 去看看
去看看


一、概述
脑胶质瘤是指起源于脑神经胶质细胞的肿瘤,是最常见的原发性颅内肿瘤,世界卫生组织(WHO)中枢神经系统肿瘤分类将脑胶质瘤分为Ⅰ-Ⅳ级,Ⅰ、Ⅱ级为低级别脑胶质瘤,Ⅲ、Ⅳ级为高级别脑胶质瘤。本规范主要涉及星形细胞、少突胶质细胞和室管膜细胞来源的高、低级别脑胶质瘤的诊治。
我国脑胶质瘤年发病率为5-8/10万,5年病死率在全身肿瘤中仅次于胰腺癌和肺癌。脑胶质瘤发病机制尚不明了,目前确定的两个危险因素是:暴露于高剂量电离辐射和与罕见综合征相关的高外显率基因遗传突变。此外,亚硝酸盐食品、病毒或细菌感染等致癌因素也可能参与脑胶质瘤的发生。
脑胶质瘤临床表现主要包括颅内压增高、神经功能及认知功能障碍和癫痫发作三大类。目前,临床诊断主要依靠计算机断层扫描(CT)及磁共振成像(MRI)检查等影像学诊断,磁共振弥散加权成像(DWI)、磁共振弥散张量成像(DTI)、磁共振灌注成像(PWI)、磁共振波谱成像(MRS)、功能磁共振成像(fMRI)、正电子发射计算机断层显像(PET)等对脑胶质瘤的鉴别诊断及治疗效果评价有重要意义。
脑胶质瘤确诊需要通过肿瘤切除或活检获取标本,进行组织和分子病理学检查,确定病理分级和分子亚型。目前主要的分子病理标记物包括:异柠檬酸脱氢酶(IDH)突变、染色体1p/19q联合缺失状态(co-deletion)、O6-甲基鸟嘌呤-DNA甲基转移酶(MGMT)启动子区甲基化、α地中海贫血伴智力低下综合征X连锁基因(ATRX)突变、端粒酶逆转录酶(TERT)启动子突变、人组蛋白H3.3(H3F3A)K27M突变、BRAF基因突变、PTPRZ1-MET基因融合、miR-181d、室管膜瘤RELA基因融合等1,2。这些分子标志物对脑胶质瘤的个体化治疗及临床预后判断具有重要意义。
脑胶质瘤治疗以手术切除为主,结合放疗、化疗等综合治疗方法。手术可以缓解临床症状,延长生存期,并获得足够肿瘤标本用以明确病理学诊断和进行分子遗传学检测。手术治疗原则是最大范围安全切除肿瘤,而常规神经导航、功能神经导航、术中神经电生理监测和术中MRI实时影像等新技术有助于实现最大范围安全切除肿瘤。放疗可杀灭或抑制肿瘤细胞,延长患者生存期,常规分割外照射是脑胶质瘤放疗的标准治疗。胶质母细胞瘤(GBM)术后放疗联合替莫唑胺(TMZ)同步并辅助化疗,已成为成人新诊断GBM的标准治疗方案。
脑胶质瘤治疗需要神经外科、神经影像科、放射治疗科、神经肿瘤科、病理科和神经康复科等多学科合作,遵循循证医学原则,采取个体化综合治疗,优化和规范治疗方案,以期达到最大治疗效益,尽可能延长患者的无进展生存期(PFS) 和总生存期(OS),提高生存质量。为使患者获得最优化的综合治疗,医师需要对患者进行密切随访观察,定期影像学复查,兼顾考虑患者的日常生活、社会和家庭活动、营养支持、疼痛控制、康复治疗和心理调控等诸多问题。
二、影像学诊断
(一)脑胶质瘤常规影像学特
神经影像常规检查目前主要包括CT和 MRI。这两种成像方法可以相对清晰精确地显示脑解剖结构特征及脑肿瘤病变形态学特征,如部位、大小、周边水肿状态、病变区域内组织均匀性、占位效应、血脑屏障破坏程度及病变造成的其他合并征象等。在图像信息上MRI优于CT。CT主要显示脑胶质瘤病变组织与正常脑组织的密度差值,特征性密度表现如钙化、出血及囊性变等,病变累及的部位,水肿状况及占位效应等;常规MRI主要显示脑胶质瘤出血、坏死、水肿组织等的不同信号强度差异及占位效应,并且可以显示病变的侵袭范围。多模态MRI不仅能反映脑胶质瘤的形态学特征,还可以体现肿瘤组织的功能及代谢状况。
常规MRI扫描,主要获取T1加权像、T2加权像、FLAIR像及进行磁共振对比剂的强化扫描。脑胶质瘤边界不清,表现为长T1、长T2信号影,信号可以不均匀,周边水肿轻重不一。因肿瘤对血脑屏障的破坏程度不同,增强扫描征象不一。
脑胶质瘤可发生于脑内各部位。低级别脑胶质瘤常规MRI呈长T1、长T2信号影,边界不清,周边轻度水肿影,局部轻度占位征象,如邻近脑室可致其轻度受压,中线移位不明显,脑池基本正常,病变区域内少见出血、坏死及囊变等表现;增强扫描显示病变极少数出现轻度异常强化影。高级别脑胶质瘤MRI信号明显不均匀,呈混杂T1/T2信号影,周边明显指状水肿影;占位征象明显,邻近脑室受压变形,中线结构移位,脑沟、脑池受压;增强扫描呈明显花环状及结节样异常强化影。
不同级别脑胶质瘤的PET成像特征各异。目前广泛使用的示踪剂为18F-FDG。低级别脑胶质瘤一般代谢活性低于正常脑灰质,高级别脑胶质瘤代谢活性可接近或高于正常脑灰质,但不同级别脑胶质瘤之间的18F-FDG代谢活性存在较大重叠(2级证据)3。氨基酸肿瘤显像具有良好的病变-本底对比度,对脑胶质瘤的分级评价优于18F-FDG,但仍存在一定重叠。
临床诊断怀疑脑胶质瘤拟行活检时,可用PET确定病变代谢活性最高的区域。18F-FET和11C-MET 比,18F-FDG具有更高的信噪比和病变对比度(2级证据)4,5。PET联合MRI检查比单独MRI检查更能准确界定放疗靶区(1级证据)6。相对于常规MRI技术,氨基酸PET可以提高勾画肿瘤生物学容积的准确度,发现潜在的被肿瘤细胞浸润/侵袭的脑组织(在常规MRI图像上可无异常发现),并将其纳入到患者的放疗靶区中(2级证据)7-9。18F-FDG PET由于肿瘤/皮层对比度较低,因而不适用于辅助制定放疗靶区(2级证据)10。
神经外科临床医师对神经影像诊断的要求很明确:首先是进行定位诊断,确定肿瘤的大小、范围、肿瘤与周围重要结构(包括重要动脉、皮层静脉、皮层功能区及神经纤维束等)的毗邻关系及形态学特征等,这对制定脑胶质瘤手术方案具有重要的作用;其次是对神经影像学提出功能状况的诊断要求,如肿瘤生长代谢、血供状态及肿瘤对周边脑组织侵袭程度等,这对患者术后的综合疗效评估具有关键作用。多模态MRI可提供肿瘤的血液动力学、代谢、神经纤维组织受累状况和皮质功能区等信息,对于脑胶质瘤的鉴别诊断、确定手术边界、预后判断、监测治疗效果及明确有无复发等具有重要意义,是形态成像诊断的一个重要补充。
表1 脑胶质瘤影像学诊断要点
| 肿瘤类型 | 影像学特征性表现 | |
| 低级别脑胶质瘤 | 主要指弥漫性星形胶质细胞瘤、少突胶质细胞瘤、少突星形胶质细胞瘤3种。特殊类型还包括:多形性黄色星形细胞瘤(PXA)、第三脑室脊索瘤样脑胶质瘤和毛细胞型星形细胞瘤等 | 弥漫性星形胶质细胞瘤MRI信号相对均匀,长T1,长T2和FLAIR高信号,多无强化;少突胶质细胞瘤表现同弥漫性星形脑胶质瘤,常伴钙化。PXA多见于颞叶,位置表浅,有囊变及壁结节。增强扫描,壁结节及邻近脑膜有强化。第三脑室脊索瘤样脑胶质瘤位于第三脑室内。毛细胞型星形细胞瘤以实性为主,常见于鞍上和小脑半球。 |
| 间变性脑胶质瘤(Ⅲ级) | 主要包括间变性星形细胞瘤、间变性少突胶质细胞瘤。 | 当MRI/CT表现似星形细胞瘤或少突胶质细胞瘤伴强化时,提示间变脑胶质瘤可能性大。 |
| Ⅳ级脑胶质瘤 | 胶质母细胞瘤;弥漫性中线胶质瘤 | 胶质母细胞瘤特征为不规则形周边强化和中央大量坏死,强化外可见水肿。弥漫中线胶质瘤常发生于丘脑、脑干等中线结构,MRI表现为长T1长T2信号,增强扫描可有不同程度的强化。 |
| 室管膜肿瘤 | 主要指Ⅱ级和Ⅲ级室管膜肿瘤。特殊类型:黏液乳头型室管膜瘤为Ⅰ级。 | 室管膜肿瘤边界清楚,多位于脑室内,信号混杂,出血、坏死、囊变和钙化可并存,瘤体强化常明显。黏液乳头型室管膜瘤好发于脊髓圆锥和马尾。 |
(二)脑胶质瘤鉴别诊断
1. 脑内转移性病变:脑内转移性病变以多发病变较为常见,多位于脑皮层下,大小不等,水肿程度不一,表现多样,多数为环状或结节样强化影。脑内转移性病变的18F-FDG代谢活性可低于、接近或高于脑灰质;氨基酸代谢活性一般高于脑灰质。单发转移癌需要与高级别脑胶质瘤鉴别,影像学上可以根据病变大小、病变累及部位、增强表现,结合病史、年龄及相关其他辅助检查结果综合鉴别。
2. 脑内感染性病变:脑内感染性病变,特别是脑脓肿,需与高级别脑胶质瘤鉴别。两者均有水肿及占位征象,强化呈环形。脑脓肿的壁常较光滑,无壁结节,而高级别脑胶质瘤多呈菜花样强化,囊内信号混杂,可伴肿瘤卒中。绝大部分高级别脑胶质瘤的氨基酸代谢活性明显高于正常脑组织,而脑脓肿一般呈低代谢。
3. 脑内脱髓鞘样病变:与脑胶质瘤易发生混淆的是肿瘤样脱髓鞘病变,增强扫描可见结节样强化影,诊断性治疗后复查,病变缩小明显,易复发,实验室检查有助于鉴别诊断。
4. 淋巴瘤:对于免疫功能正常的患者,淋巴瘤的MRI信号多较均匀,瘤内出血及坏死少见,增强呈明显均匀强化。18F-FDG代谢活性一般较高级别脑胶质瘤高且代谢分布较均匀。
5. 其他神经上皮来源肿瘤:包括中枢神经细胞瘤等。可以根据肿瘤发生部位、增强表现进行初步鉴别诊断。
(三)脑胶质瘤影像学分级
1. 常规MRI检查:除部分Ⅱ级脑胶质瘤(如多形性黄色星形细胞瘤、第三脑室脊索瘤样脑胶质瘤和室管膜瘤等)外,高级别脑胶质瘤MRI常有强化伴卒中、坏死及囊变。MRI有无强化及强化程度受到诸多因素影响,如使用激素、注射对比剂的量、机器型号及扫描技术等。
2. 多模态MRI 检查:包括DWI、PWI及MRS等。DWI高信号区域,提示细胞密度大,代表高级别病变区;PWI高灌注区域,提示血容量增多,多为高级别病变区;MRS中Cho和Cho/NAA比值升高,与肿瘤级别正相关。
3. 18F-FDG PET:脑胶质瘤代谢成像的肿瘤-本底对比度偏低,而氨基酸肿瘤显像具有较好的组织对比度,因此建议采用氨基酸PET脑显像评价脑胶质瘤级别(2级证据)11,12。11C-MET PET评估准确度高于MRI,高级别脑胶质瘤的11C-MET代谢活性通常高于低级别脑胶质瘤,但高/低级别脑胶质瘤间仍存在一定的重叠(2级证据)13-17。必要时建议使用18F-FDG PET动态成像分析以提高对脑胶质瘤的影像学分级。
(四)脑胶质瘤治疗后影像学评估
脑胶质瘤术后24-72小时内需复查MRI(平扫+增强),评估肿瘤切除程度,并以此作为脑胶质瘤术后基线影像学资料,用于后续比对。胶质瘤治疗效果的影像学评价参见RANO标准18(表2)。
表2 脑胶质瘤治疗效果评估RANO标准
| 完全缓解 (CR) | 部分缓解 (PR) | 疾病稳定 (SD) | 疾病进展 (PD) | |
| T1增强 | 无 | 缩小≥50% | 变化在-50%至+25%之间 | 增加≥25% |
| T2/FLAIR | 稳定或减小 | 稳定或减小 | 稳定或减小 | 增加 |
| 新发病变 | 无 | 无 | 无 | 有 |
| 激素使用 | 无 | 稳定或减少 | 稳定或减少 | 不适用* |
| 临床症状 | 稳定或改善 | 稳定或改善 | 稳定或改善 | 恶化 |
| 需要满足条件 | 以上全部 | 以上全部 | 以上全部 | 任意一项 |
*在出现持续的临床症状恶化时,即为疾病进展,但不能单纯的将激素用量增加作为疾病进展的依据。
脑胶质瘤按照复发部位包括原位复发、远处复发和脊髓播散等特殊方式,其中以原位复发最为多见19。组织病理学诊断仍然是金标准。假性进展多见于放/化疗后3个月内,少数患者可见于10-18个月内。常表现为病变周边的环形强化,水肿明显,有占位征象,需要结合临床谨慎判断。对于高级别脑胶质瘤,氨基酸PET对鉴别治疗相关变化(假性进展、放射性坏死)和肿瘤复发/进展的准确度较高(2级证据)20-22。放射性坏死多见于放疗3个月后,目前尚无特异性检查手段鉴别放射性坏死与肿瘤进展/复发。对于高级别胶质瘤,18F-FDG PET用于评价术后肿瘤复发和放射性坏死较MRI优势不明显,氨基酸PET用于鉴别肿瘤进展和治疗相关反应具有较高的敏感度和特异度。对于低级别胶质瘤,18F-FDG PET不适用于评价肿瘤治疗反应,而氨基酸PET的评价作用也有限(1级证据)23-25。定期MRI或PET检查,有助于鉴别假性进展和肿瘤进展/复发(表3)。
表3 脑胶质瘤复发、假性进展及放射性坏死鉴别方法
| 项目 | 肿瘤复发 | 假性进展 | 放射性坏死 |
| 发生时间 | 任何时间 | 多见于放/化疗后3个月内,少数患者可见于10个月内 | 治疗后数月至数年 |
| 临床症状 | 恶化 | 不变或恶化 | 不变或恶化 |
| MRI增强扫描 | 多病变和胼胝体受侵通常是复发 | 大片长T1和T2信号,内有不规则的强化,占位效应明显。 | MRI增强扫描可见强化,晚期表现为高信号 |
| PWI | 通常髙灌注 | 通常低灌注 | 通常低灌注 |
| MRS | Cho/NAA,Cho/Cr较高 | Cho/NAA,Cho/Cr较低 | Cho/NAA,Cho/Cr较低 |
| DWI | 弥散受限 | 比肿瘤信号低 | 比肿瘤信号低 |
| 葡萄糖PET | 通常高代谢 | 高代谢或低代谢 | 低代谢 |
| 氨基酸PET和18F-FDGPET | 高代谢 | 低代谢 | 低代谢 |
| 好发因素 | RT+TMZ | RT | |
| 与放疗关系 | 可在放射治疗野范围外 | 多在放射治疗野范围内 | 多在放射治疗野范围内 |
| 发生率 | 几乎全部 | 总20%-30%,在同步放化疗中常见,特别是MGMT启动子区甲基化者发生率更高 | 与剂量有关,大约在2%-18% |
三、神经病理学与分子病理学诊断
(一)2016版WHO中枢神经系统肿瘤分类标准
| 肿瘤分类 | WHO分级 | ICD-O编码 |
| 弥漫性星形细胞和少突胶质细胞肿瘤 | ||
| 弥漫性星形细胞瘤,IDH突变型 | II | 9400/3 |
| 肥胖细胞型星形细胞瘤,IDH突变型 | 9411/3 | |
| 弥漫性星形细胞瘤,IDH野生型 | II | 9400/3 |
| 弥漫性星形细胞瘤,NOS | II | 9400/3 |
| 间变性星形细胞瘤,IDH突变型 | III | 9401/3 |
| 间变性星形细胞瘤,IDH野生型 | III | 9401/3 |
| 间变性星形细胞瘤,NOS | III | 9401/3 |
| 胶质母细胞瘤,IDH野生型 | IV | 9440/3 |
| 巨细胞型胶质母细胞瘤 | 9441/3 | |
| 胶质肉瘤 | 9442/3 | |
| 上皮样胶质母细胞瘤 | 9440/3 | |
| 胶质母细胞瘤,IDH突变型 | IV | 9445/3 |
| 胶质母细胞瘤,NOS | IV | 9440/3 |
| 弥漫性中线胶质瘤,H3 K27M突变型 | IV | 9385/3 |
| 少突胶质细胞瘤,IDH突变和1p/19q联合缺失型 | II | 9450/3 |
| 少突胶质细胞瘤,NOS | II | 9450/3 |
| 间变性少突胶质细胞瘤,IDH突变和1p/19q联合缺失型 | III | 9451/3 |
| 间变性少突胶质细胞瘤,NOS | III | 9451/3 |
| 少突星形细胞瘤,NOS | II | 9382/3 |
| 间变性少突星形细胞瘤,NOS | III | 9382/3 |
| 其他星形细胞肿瘤 | ||
| 毛细胞型星形细胞瘤 | I | 9421/1 |
| 毛黏液样型星形细胞瘤 | 9425/3 | |
| 室管膜下巨细胞型星形细胞瘤 | I | 9384/1 |
| 多形性黄色星形细胞瘤 | II | 9424/3 |
| 间变性多形性黄色星形细胞瘤 | III | 9424/3 |
| 室管膜肿瘤 | ||
| 室管膜下瘤 | I | 9383/1 |
| 黏液乳头型室管膜瘤 | I | 9394/1 |
| 室管膜瘤 | II | 9391/3 |
| 乳头型室管膜瘤 | 9393/3 | |
| 透明细胞型室管膜瘤 | 9391/3 | |
| 伸长细胞型室管膜瘤 | 9391/3 | |
| 室管膜瘤,RELA融合基因阳性 | II/III | 9396/3 |
| 间变性室管膜瘤 | III | 9392/3 |
| 其他脑胶质瘤 | ||
| 第三脑室脊索样型脑胶质瘤 | II | 9444/1 |
| 血管中心型脑胶质瘤 | I | 9431/1 |
| 星形母细胞瘤 | 9430/3 |
(二)脑胶质瘤病理学综合诊断
脑胶质瘤是一组具有胶质细胞表型特征的神经上皮肿瘤的总称,2016年世界卫生组织发布了第四版《中枢神经系统肿瘤WHO分类》(修订版),首次整合了肿瘤的组织学特征和分子表型,提出了新的肿瘤分类标准。这一标准是目前脑胶质瘤诊断及分级的重要依据。
1. 肿瘤组织学分类与分子表型
(1)星形细胞肿瘤
① 弥漫性星形细胞瘤,IDH突变型
定义 以 IDH1或 IDH2基因突变为特征,可伴有 TP53及 ATRX基因突变。细胞分化程度较高,生长缓慢。可发生于中枢神经系统任何部位,额叶多见;肿瘤具有恶变潜能,可进展成 IDH突变型间变性星形细胞瘤,甚或 IDH突变型GBM。
大体 肿瘤边界不清,位于灰质或白质内,可见大小不等的囊腔、颗粒样区域及软硬度不同的区域。
镜下 肿瘤由分化好的纤维型星形细胞组成,细胞密度中等,核不典型,核分裂像少或缺如。间质疏松,常伴微囊形成,不伴有血管内皮细胞增生。Ki-67增殖指数常小于4%。
免疫组织化学 胶质纤维酸性蛋白(GFAP)、波形蛋白(Vimentin)、Ki-67/MIB-1、p53蛋白、IDH1 R132H和ATRX。
分子病理学 IDH1codon 132、IDH2codon 172基因突变。
肥胖细胞型星形细胞瘤,IDH突变型
定义 是弥漫性星形细胞瘤,IDH突变型的一个亚型,以含有大量肥胖型星形细胞为特点,且肥胖型星形细胞含量大于20%。
大体 与其他低级别弥漫性脑胶质瘤无区别。
镜下 肿瘤细胞呈多角形,胞质丰富、嗜酸性、毛玻璃样,核常偏位,染色质簇状??
1. ADDIN EN.REFLIST1. 中国脑胶质瘤协作组. 中国脑胶质瘤分子诊疗指南. 中华神经外科杂志 2014;30:435-444.
2. Jiang T, Mao Y, Ma W, et al. CGCG clinical practice guidelines for the management of adult diffuse gliomas. Cancer letters 2016;375:263-273.
3. Dunet V, Pomoni A, Hottinger A, et al. Performance of 18F-FET versus 18F-FDG-PET for the diagnosis and grading of brain tumors: systematic review and meta-analysis. Neuro-oncology 2016;18:426-434.
4. Kunz M, Thon N, Eigenbrod S, et al. Hot spots in dynamic (18)FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro-oncology 2011;13:307-316.
5. Pafundi DH, Laack NN, Youland RS, et al. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neuro-oncology 2013;15:1058-1067.
6. Weller M, van den Bent M, Hopkins K, et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol 2014;15:e395-403.
7. Grosu AL, Weber WA, Franz M, et al. Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys 2005;63:511-519.
8. Miwa K, Matsuo M, Ogawa S, et al. Re-irradiation of recurrent glioblastoma multiforme using 11C-methionine PET/CT/MRI image fusion for hypofractionated stereotactic radiotherapy by intensity modulated radiation therapy. Radiat Oncol 2014;9:181.
9. Weber DC, Casanova N, Zilli T, et al. Recurrence pattern after [(18)F]fluoroethyltyrosine-positron emission tomography-guided radiotherapy for high-grade glioma: a prospective study. Radiother Oncol 2009;93:586-592.
10. Tralins KS, Douglas JG, Stelzer KJ, et al. Volumetric analysis of 18F-FDG PET in glioblastoma multiforme: prognostic information and possible role in definition of target volumes in radiation dose escalation. J Nucl Med 2002;43:1667-1673.
11. Giammarile F, Cinotti LE, Jouvet A, et al. High and low grade oligodendrogliomas (ODG): correlation of amino-acid and glucose uptakes using PET and histological classifications. Journal of neuro-oncology 2004;68:263-274.
12. Manabe O, Hattori N, Yamaguchi S, et al. Oligodendroglial component complicates the prediction of tumour grading with metabolic imaging. Eur J Nucl Med Mol Imaging 2015;42:896-904.
13. Hutterer M, Nowosielski M, Putzer D, et al. [18F]-fluoro-ethyl-L-tyrosine PET: a valuable diagnostic tool in neuro-oncology, but not all that glitters is glioma. Neuro-oncology 2013;15:341-351.
14. Jansen NL, Schwartz C, Graute V, et al. Prediction of oligodendroglial histology and LOH 1p/19q using dynamic [(18)F]FET-PET imaging in intracranial WHO grade II and III gliomas. Neuro-oncology 2012;14:1473-1480.
15. Moulin-Romsee G, D'Hondt E, de Groot T, et al. Non-invasive grading of brain tumours using dynamic amino acid PET imaging: does it work for 11C-methionine? Eur J Nucl Med Mol Imaging 2007;34:2082-2087.
16. Popperl G, Kreth FW, Mehrkens JH, et al. FET PET for the evaluation of untreated gliomas: correlation of FET uptake and uptake kinetics with tumour grading. Eur J Nucl Med Mol Imaging 2007;34:1933-1942.
17. Rapp M, Heinzel A, Galldiks N, et al. Diagnostic performance of 18F-FET PET in newly diagnosed cerebral lesions suggestive of glioma. J Nucl Med 2013;54:229-235.
18. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010;28:1963-1972.
19. Brandes AA, Tosoni A, Franceschi E, et al. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation With MGMT promoter methylation status. J Clin Oncol 2009;27:1275-1279.
20. Chen W, Silverman DH, Delaloye S, et al. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med 2006;47:904-911.
21. Herrmann K, Czernin J, Cloughesy T, et al. Comparison of visual and semiquantitative analysis of 18F-FDOPA-PET/CT for recurrence detection in glioblastoma patients. Neuro-oncology 2014;16:603-609.
22. Walter F, Cloughesy T, Walter MA, et al. Impact of 3,4-dihydroxy-6-18F-fluoro-L-phenylalanine PET/CT on managing patients with brain tumors: the referring physician's perspective. J Nucl Med 2012;53:393-398.
23. Albert NL, Weller M, Suchorska B, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. Neuro-oncology 2016;18:1199-1208.
24. Voges J, Herholz K, Holzer T, et al. 11C-methionine and 18F-2-fluorodeoxyglucose positron emission tomography: a tool for diagnosis of cerebral glioma and monitoring after brachytherapy with 125I seeds. Stereotact Funct Neurosurg 1997;69:129-135.
25. Wyss M, Hofer S, Bruehlmeier M, et al. Early metabolic responses in temozolomide treated low-grade glioma patients. Journal of neuro-oncology 2009;95:87-93.
26. Singh D, Chan JM, Zoppoli P, et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 2012;337:1231-1235.
27. Zhang W, Zhang J, Hoadley K, et al. miR-181d: a predictive glioblastoma biomarker that downregulates MGMT expression. Neuro-oncology 2012;14:712-719.
28. Bao ZS, Chen HM, Yang MY, et al. RNA-seq of 272 gliomas revealed a novel, recurrent PTPRZ1-MET fusion transcript in secondary glioblastomas. Genome research 2014;24:1765-1773.
29. Wu JS, Zhou LF, Tang WJ, et al. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: a prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery 2007;61:935-948; discussion 948-939.
30. Duffau H. Is supratotal resection of glioblastoma in noneloquent areas possible? World neurosurgery 2014;82:e101-103.
31. Wu JS, Gong X, Song YY, et al. 3.0-T intraoperative magnetic resonance imaging-guided resection in cerebral glioma surgery: interim analysis of a prospective, randomized, triple-blind, parallel-controlled trial. Neurosurgery 2014;61 Suppl 1:145-154.
32. Kumar A, Chandra PS, Sharma BS, et al. The role of neuronavigation-guided functional MRI and diffusion tensor tractography along with cortical stimulation in patients with eloquent cortex lesions. Br J Neurosurg 2014;28:226-233.
33. Bello L, Gambini A, Castellano A, et al. Motor and language DTI Fiber Tracking combined with intraoperative subcortical mapping for surgical removal of gliomas. NeuroImage 2008;39:369-382.
34. Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 2006;7:392-401.
35. Pignatti F, van den Bent M, Curran D, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol 2002;20:2076-2084.
36. Ahmadi R, Dictus C, Hartmann C, et al. Long-term outcome and survival of surgically treated supratentorial low-grade glioma in adult patients. Acta Neurochir (Wien) 2009;151:1359-1365.
37. Ius T, Isola M, Budai R, et al. Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients: clinical article. Journal of neurosurgery 2012;117:1039-1052.
38. Simon M, Neuloh G, von Lehe M, et al. Insular gliomas: the case for surgical management. J Neurosurg 2009;110:685-695.
39. Zinn PO, Colen RR, Kasper EM, et al. Extent of resection and radiotherapy in GBM: A 1973 to 2007 surveillance, epidemiology and end results analysis of 21,783 patients. Int J Oncol 2013;42:929-934.
40. Kreth FW, Thon N, Simon M, et al. Gross total but not incomplete resection of glioblastoma prolongs survival in the era of radiochemotherapy. Ann Oncol 2013;24:3117-3123.
41. Laws ER, Parney IF, Huang W, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg 2003;99:467-473.
42. Sanai N, Polley MY, McDermott MW, et al. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 2011;115:3-8.
43. McGirt MJ, Chaichana KL, Attenello FJ, et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery 2008;63:700-707; author reply 707-708.
44. Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 2008;26:1338-1345.
45. 中国脑胶质瘤协作组. 成人幕上低级别胶质瘤的手术治疗指南. 中华神经外科杂志 2016;32:652-658.
46. Montemurro N, Perrini P, Blanco MO, et al. Second surgery for recurrent glioblastoma: A concise overview of the current literature. Clin Neurol Neurosurg 2016;142:60-64.
47. Robin AM, Lee I, Kalkanis SN. Reoperation for Recurrent Glioblastoma Multiforme. Neurosurg Clin N Am 2017;28:407-428.
48. Chang EF, Clark A, Smith JS, et al. Functional mapping-guided resection of low-grade gliomas in eloquent areas of the brain: improvement of long-term survival. Clinical article. J Neurosurg 2011;114:566-573.
49. 张忠, 江涛, 谢坚, et al. 术中功能定位切除辅助运动区低级别胶质瘤. 中华神经外科杂志 2008;24:35-38.
50. 张忠, 江涛, 谢坚, et al. 唤醒麻醉和术中功能定位切除语言区胶质瘤. 中华神经外科杂志 2007;23:643-645.
51. Ius T, Angelini E, Thiebaut de Schotten M, et al. Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: towards a "minimal common brain". NeuroImage 2011;56:992-1000.
52. Yordanova YN, Moritz-Gasser S, Duffau H. Awake surgery for WHO Grade II gliomas within "noneloquent" areas in the left dominant hemisphere: toward a "supratotal" resection. Clinical article. Journal of neurosurgery 2011;115:232-239.
53. 白红民, 江涛, 王伟民, et al. 类别特异性命名区脑定位的临床研究. 中华神经外科杂志 2010;26:1067-1070.
54. Wang X, Wang YY, Jiang T, et al. Direct evidence of the left caudate's role in bilingual control: an intra-operative electrical stimulation study. Neurocase 2013;19:462-469.
55. Hervey-Jumper SL, Li J, Lau D, et al. Awake craniotomy to maximize glioma resection: methods and technical nuances over a 27-year period. Journal of neurosurgery 2015;123:325-339.
56. Jingshan L, Shengyu F, Xing F, et al. Morphometry of the Hand Knob Region and Motor Function Change in Eloquent Area Glioma Patients. Clin Neuroradiol 2018.
57. Moller M, Freund M, Greiner C, et al. Real time fMRI: a tool for the routine presurgical localisation of the motor cortex. Eur Radiol 2005;15:292-295.
58. Xie J, Chen XZ, Jiang T, et al. Preoperative blood oxygen level-dependent functional magnetic resonance imaging in patients with gliomas involving the motor cortical areas. Chinese medical journal 2008;121:631-635.
59. Belliveau JW, Kennedy DN, Jr., McKinstry RC, et al. Functional mapping of the human visual cortex by magnetic resonance imaging. Science 1991;254:716-719.
60. Fang S, Liang J, Qian T, et al. Anatomic Location of Tumor Predicts the Accuracy of Motor Function Localization in Diffuse Lower-Grade Gliomas Involving the Hand Knob Area. AJNR Am J Neuroradiol 2017;38:1990-1997.
61. Hall WA, Liu H, Truwit CL. Functional magnetic resonance imaging-guided resection of low-grade gliomas. Surgical neurology 2005;64:20-27; discussion 27.
62. Jack CR, Jr., Thompson RM, Butts RK, et al. Sensory motor cortex: correlation of presurgical mapping with functional MR imaging and invasive cortical mapping. Radiology 1994;190:85-92.
63. Tarapore PE, Martino J, Guggisberg AG, et al. Magnetoencephalographic imaging of resting-state functional connectivity predicts postsurgical neurological outcome in brain gliomas. Neurosurgery 2012;71:1012-1022.
64. Qiu TM, Yan CG, Tang WJ, et al. Localizing hand motor area using resting-state fMRI: validated with direct cortical stimulation. Acta neurochirurgica 2014;156:2295-2302.
65. Gunnarsson T, Olafsson E, Sighvatsson V, et al. Surgical treatment of patients with low-grade astrocytomas and medically intractable seizures. Acta neurologica Scandinavica 2002;105:289-292.
66. 中国脑胶质瘤协作组. 唤醒状态下切除脑功能区胶质瘤手术技术指南(2014版). 中国微侵袭神经外科杂志 2014;19:479-485.
67. Du G, Zhou L, Mao Y. Neuronavigator-guided glioma surgery. Chin Med J (Engl) 2003;116:1484-1487.
68. Nimsky C, Ganslandt O, Merhof D, et al. Intraoperative visualization of the pyramidal tract by diffusion-tensor-imaging-based fiber tracking. Neuroimage 2006;30:1219-1229.
69. Nimsky C, Ganslandt O, Buchfelder M, et al. Intraoperative visualization for resection of gliomas: the role of functional neuronavigation and intraoperative 1.5 T MRI. Neurological research 2006;28:482-487.
70. 吴劲松, 毛颖, 姚成军, et al. 术中磁共振影像神经导航治疗脑胶质瘤的临床初步应用(附61例分析). 中国微侵袭神经外科杂志 2007;12:105-109.
71. Duffau H. Surgery of low-grade gliomas: towards a 'functional neurooncology'. Curr Opin Oncol 2009;21:543-549.
72. Bello L, Gallucci M, Fava M, et al. Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery 2007;60:67-80; discussion 80-62.
73. Kim SS, McCutcheon IE, Suki D, et al. Awake craniotomy for brain tumors near eloquent cortex: correlation of intraoperative cortical mapping with neurological outcomes in 309 consecutive patients. Neurosurgery 2009;64:836-845; discussion 345-836.
74. De Benedictis A, Moritz-Gasser S, Duffau H. Awake mapping optimizes the extent of resection for low-grade gliomas in eloquent areas. Neurosurgery 2010;66:1074-1084; discussion 1084.
75. Talacchi A, Turazzi S, Locatelli F, et al. Surgical treatment of high-grade gliomas in motor areas. The impact of different supportive technologies: a 171-patient series. Journal of neuro-oncology 2010;100:417-426.
76. Tate MC, Herbet G, Moritz-Gasser S, et al. Probabilistic map of critical functional regions of the human cerebral cortex: Broca's area revisited. Brain 2014;137:2773-2782.
77. Roux FE, Dufor O, Lauwers-Cances V, et al. Electrostimulation mapping of spatial neglect. Neurosurgery 2011;69:1218-1231.
78. Ilmberger J, Ruge M, Kreth FW, et al. Intraoperative mapping of language functions: a longitudinal neurolinguistic analysis. J Neurosurg 2008;109:583-592.
79. Duffau H, Capelle L, Sichez N, et al. Intraoperative mapping of the subcortical language pathways using direct stimulations. An anatomo-functional study. Brain 2002;125:199-214.
80. Magill ST, Han SJ, Li J, et al. Resection of primary motor cortex tumors: feasibility and surgical outcomes. Journal of neurosurgery 2017:1-12.
81. Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008;62:753-764; discussion 264-756.
82. Sanai N, Berger MS. Operative techniques for gliomas and the value of extent of resection. Neurotherapeutics 2009;6:478-486.
83. Zhang Z, Jiang T, Xie J, et al. Surgical strategies for glioma involving language areas. Chinese medical journal 2008;121:1800-1805.
84. Lima GLO, Dezamis E, Corns R, et al. Surgical resection of incidental diffuse gliomas involving eloquent brain areas. Rationale, functional, epileptological and oncological outcomes. Neurochirurgie 2017;63:250-258.
85. Xia L, Fang C, Chen G, et al. Relationship between the extent of resection and the survival of patients with low-grade gliomas: a systematic review and meta-analysis. BMC Cancer 2018;18:48.
86. Jiang B, Chaichana K, Veeravagu A, et al. Biopsy versus resection for the management of low-grade gliomas. Cochrane Database Syst Rev 2017;4:CD009319.
87. Englot DJ, Han SJ, Berger MS, et al. Extent of surgical resection predicts seizure freedom in low-grade temporal lobe brain tumors. Neurosurgery 2012;70:921-928; discussion 928.
88. You G, Sha ZY, Yan W, et al. Seizure characteristics and outcomes in 508 Chinese adult patients undergoing primary resection of low-grade gliomas: a clinicopathological study. Neuro-oncology 2012;14:230-241.
89. Clusmann H, Kral T, Gleissner U, et al. Analysis of different types of resection for pediatric patients with temporal lobe epilepsy. Neurosurgery 2004;54:847-859; discussion 859-860.
90. Wang YY, Zhang T, Li SW, et al. Mapping p53 mutations in low-grade glioma: a voxel-based neuroimaging analysis. AJNR American journal of neuroradiology 2015;36:70-76.
91. Zaatreh MM, Spencer DD, Thompson JL, et al. Frontal lobe tumoral epilepsy: clinical, neurophysiologic features and predictors of surgical outcome. Epilepsia 2002;43:727-733.
92. Wray CD, McDaniel SS, Saneto RP, et al. Is postresective intraoperative electrocorticography predictive of seizure outcomes in children? J Neurosurg Pediatr 2012;9:546-551.
93. Yao PS, Zheng SF, Wang F, et al. Surgery guided with intraoperative electrocorticography in patients with low-grade glioma and refractory seizures. Journal of neurosurgery 2018;128:840-845.
94. Pereira LC, Oliveira KM, L'Abbate GL, et al. Outcome of fully awake craniotomy for lesions near the eloquent cortex: analysis of a prospective surgical series of 79 supratentorial primary brain tumors with long follow-up. Acta Neurochir (Wien) 2009;151:1215-1230.
95. Lima GL, Duffau H. Is there a risk of seizures in "preventive" awake surgery for incidental diffuse low-grade gliomas? Journal of neurosurgery 2015;122:1397-1405.
96. Boetto J, Bertram L, Moulinie G, et al. Low Rate of Intraoperative Seizures During Awake Craniotomy in a Prospective Cohort with 374 Supratentorial Brain Lesions: Electrocorticography Is Not Mandatory. World neurosurgery 2015;84:1838-1844.
97. Vecht CJ, Kerkhof M, Duran-Pena A. Seizure prognosis in brain tumors: new insights and evidence-based management. The oncologist 2014;19:751-759.
98. Di Bonaventura C, Albini M, D'Elia A, et al. Epileptic seizures heralding a relapse in high grade gliomas. Seizure 2017;51:157-162.
99. Kahlenberg CA, Fadul CE, Roberts DW, et al. Seizure prognosis of patients with low-grade tumors. Seizure 2012;21:540-545.
100. Sun MZ, Oh T, Ivan ME, et al. Survival impact of time to initiation of chemoradiotherapy after resection of newly diagnosed glioblastoma. Journal of neurosurgery 2015;122:1144-1150.
101. Merchant TE, Kun LE, Wu S, et al. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol 2009;27:3598-3604.
102. Cabrera AR, Kirkpatrick JP, Fiveash JB, et al. Radiation therapy for glioblastoma: Executive summary of an American Society for Radiation Oncology Evidence-Based Clinical Practice Guideline. Pract Radiat Oncol 2016;6:217-225.
103. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Central Nervous System Cancers (Version 1.2018). 2018.
104. Chiesa S, et al. PD-0516: Edema or not edema: this the matter in glioblastoma CTV! Hypothesis from two sequential phase II studies. Radiotherapy and Oncology 2014;111:S203-S204.
105. Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 2013;31:4085-4091.
106. Chang EL, Akyurek S, Avalos T, et al. Evaluation of peritumoral edema in the delineation of radiotherapy clinical target volumes for glioblastoma. Int J Radiat Oncol Biol Phys 2007;68:144-150.
107. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine 2005;352:987-996.
108. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. The New England journal of medicine 2005;352:997-1003.
109. van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol 2013;31:344-350.
110. Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol 2013;31:337-343.
111. Intergroup Radiation Therapy Oncology Group T, Cairncross G, Berkey B, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol 2006;24:2707-2714.
112. Yang P, Cai J, Yan W, et al. Classification based on mutations of TERT promoter and IDH characterizes subtypes in grade II/III gliomas. Neuro-oncology 2016;18:1099-1108.
113. Daniels TB, Brown PD, Felten SJ, et al. Validation of EORTC prognostic factors for adults with low-grade glioma: a report using intergroup 86-72-51. Int J Radiat Oncol Biol Phys 2011;81:218-224.
114. Smith JS, Perry A, Borell TJ, et al. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol 2000;18:636-645.
115. Nabors LB, Portnow J, Ammirati M, et al. NCCN Guidelines Insights: Central Nervous System Cancers, Version 1.2017. J Natl Compr Canc Netw 2017;15:1331-1345.
116. Shaw EG, Berkey B, Coons SW, et al. Recurrence following neurosurgeon-determined gross-total resection of adult supratentorial low-grade glioma: results of a prospective clinical trial. Journal of neurosurgery 2008;109:835-841.
117. Karim AB, Maat B, Hatlevoll R, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys 1996;36:549-556.
118. Shaw E, Arusell R, Scheithauer B, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol 2002;20:2267-2276.
119. Klein M, Heimans JJ, Aaronson NK, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet 2002;360:1361-1368.
120. Mansur DB, Perry A, Rajaram V, et al. Postoperative radiation therapy for grade II and III intracranial ependymoma. Int J Radiat Oncol Biol Phys 2005;61:387-391.
121. Taylor RE. Review of radiotherapy dose and volume for intracranial ependymoma. Pediatr Blood Cancer 2004;42:457-460.
122. Rodriguez D, Cheung MC, Housri N, et al. Outcomes of malignant CNS ependymomas: an examination of 2408 cases through the Surveillance, Epidemiology, and End Results (SEER) database (1973-2005). J Surg Res 2009;156:340-351.
123. Swanson EL, Amdur RJ, Morris CG, et al. Intracranial ependymomas treated with radiotherapy: long-term results from a single institution. Journal of neuro-oncology 2011;102:451-457.
124. Vanuytsel LJ, Bessell EM, Ashley SE, et al. Intracranial ependymoma: long-term results of a policy of surgery and radiotherapy. Int J Radiat Oncol Biol Phys 1992;23:313-319.
125. Merchant TE, Li C, Xiong X, et al. Conformal radiotherapy after surgery for paediatric ependymoma: a prospective study. Lancet Oncol 2009;10:258-266.
126. 《中国中枢神经系统胶质瘤诊断与治疗指南》编写组. 中国中枢神经系统胶质瘤诊断与治疗指南(2015). 中华医学杂志 2016;96:485-509.
127. Fogh SE, Andrews DW, Glass J, et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol 2010;28:3048-3053.
128. Cabrera AR, Cuneo KC, Desjardins A, et al. Concurrent stereotactic radiosurgery and bevacizumab in recurrent malignant gliomas: a prospective trial. Int J Radiat Oncol Biol Phys 2013;86:873-879.
129. Lawrence YR, Li XA, el Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys 2010;76:S20-27.
130. Minniti G, Armosini V, Salvati M, et al. Fractionated stereotactic reirradiation and concurrent temozolomide in patients with recurrent glioblastoma. Journal of neuro-oncology 2011;103:683-691.
131. Boothe D, Young R, Yamada Y, et al. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro-oncology 2013;15:1257-1263.
132. Minniti G, Agolli L, Falco T, et al. Hypofractionated stereotactic radiotherapy in combination with bevacizumab or fotemustine for patients with progressive malignant gliomas. Journal of neuro-oncology 2015;122:559-566.
133. Cuneo KC, Vredenburgh JJ, Sampson JH, et al. Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys 2012;82:2018-2024.
134. Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol 2009;8:810-818.
135. Postma TJ, Klein M, Verstappen CC, et al. Radiotherapy-induced cerebral abnormalities in patients with low-grade glioma. Neurology 2002;59:121-123.
136. Chamberlain MC, Glantz MJ, Chalmers L, et al. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. Journal of neuro-oncology 2007;82:81-83.
137. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459-466.
138. Wolff JE, Berrak S, Koontz Webb SE, et al. Nitrosourea efficacy in high-grade glioma: a survival gain analysis summarizing 504 cohorts with 24193 patients. Journal of neuro-oncology 2008;88:57-63.
139. van den Bent MJ, Baumert B, Erridge SC, et al. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet 2017;390:1645-1653.
140. Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol 2004;22:1583-1588.
141. Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol 2012;13:916-926.
142. Gornet MK, Buckner JC, Marks RS, et al. Chemotherapy for advanced CNS ependymoma. Journal of neuro-oncology 1999;45:61-67.
143. Ruda R, Bosa C, Magistrello M, et al. Temozolomide as salvage treatment for recurrent intracranial ependymomas of the adult: a retrospective study. Neuro-oncology 2016;18:261-268.
144. Shaw EG, Wang M, Coons SW, et al. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol 2012;30:3065-3070.
145. Pouratian N, Gasco J, Sherman JH, et al. Toxicity and efficacy of protracted low dose temozolomide for the treatment of low grade gliomas. Journal of neuro-oncology 2007;82:281-288.
146. Kesari S, Schiff D, Drappatz J, et al. Phase II study of protracted daily temozolomide for low-grade gliomas in adults. Clin Cancer Res 2009;15:330-337.
147. Nicholson HS, Kretschmar CS, Krailo M, et al. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: a report from the Children's Oncology Group. Cancer 2007;110:1542-1550.
148. Perry JR, Rizek P, Cashman R, et al. Temozolomide rechallenge in recurrent malignant glioma by using a continuous temozolomide schedule: the "rescue" approach. Cancer 2008;113:2152-2157.
149. Triebels VH, Taphoorn MJ, Brandes AA, et al. Salvage PCV chemotherapy for temozolomide-resistant oligodendrogliomas. Neurology 2004;63:904-906.
150. Massimino M, Spreafico F, Riva D, et al. A lower-dose, lower-toxicity cisplatin-etoposide regimen for childhood progressive low-grade glioma. Journal of neuro-oncology 2010;100:65-71.
151. Moghrabi A, Friedman HS, Ashley DM, et al. Phase II study of carboplatin (CBDCA) in progressive low-grade gliomas. Neurosurg Focus 1998;4:e3.
152. Brandes AA, Basso U, Vastola F, et al. Carboplatin and teniposide as third-line chemotherapy in patients with recurrent oligodendroglioma or oligoastrocytoma: a phase II study. Ann Oncol 2003;14:1727-1731.
153. Yung WK, Prados MD, Yaya-Tur R, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol 1999;17:2762-2771.
154. Perry JR, Belanger K, Mason WP, et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol 2010;28:2051-2057.
155. Wick W, Puduvalli VK, Chamberlain MC, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol 2010;28:1168-1174.
156. Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 2008;70:779-787.
157. Chamberlain MC, Johnston S. Bevacizumab for recurrent alkylator-refractory anaplastic oligodendroglioma. Cancer 2009;115:1734-1743.
158. Chamberlain MC, Johnston S. Salvage chemotherapy with bevacizumab for recurrent alkylator-refractory anaplastic astrocytoma. Journal of neuro-oncology 2009;91:359-367.
159. Taillibert S, Vincent LA, Granger B, et al. Bevacizumab and irinotecan for recurrent oligodendroglial tumors. Neurology 2009;72:1601-1606.
160. Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 2007;13:1253-1259.
161. Soffietti R RR, Trevisan E, et al. Phase II study of bevacizumab and nitrosourea in patients with recurrent malignant glioma: A multicenter Italian study [abstract]. J Clin Oncol 2009;27:2012.
162. Mrugala MM, Crew LK, Fink JR, et al. Carboplatin and bevacizumab for recurrent malignant glioma. Oncol Lett 2012;4:1082-1086.
163. Thompson EM, Dosa E, Kraemer DF, et al. Treatment with bevacizumab plus carboplatin for recurrent malignant glioma. Neurosurgery 2010;67:87-93.
164. Chamberlain MC, Wei-Tsao DD, Blumenthal DT, et al. Salvage chemotherapy with CPT-11 for recurrent temozolomide-refractory anaplastic astrocytoma. Cancer 2008;112:2038-2045.
165. Chamberlain MC. Salvage chemotherapy with CPT-11 for recurrent oligodendrogliomas. Journal of neuro-oncology 2002;59:157-163.
166. Chamberlain MC, Tsao-Wei DD. Salvage chemotherapy with cyclophosphamide for recurrent, temozolomide-refractory glioblastoma multiforme. Cancer 2004;100:1213-1220.
167. Chamberlain MC, Tsao-Wei DD, Groshen S. Salvage chemotherapy with cyclophosphamide for recurrent temozolomide-refractory anaplastic astrocytoma. Cancer 2006;106:172-179.
168. Fulton D, Urtasun R, Forsyth P. Phase II study of prolonged oral therapy with etoposide (VP16) for patients with recurrent malignant glioma. Journal of neuro-oncology 1996;27:149-155.
169. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27:4733-4740.
170. Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 2009;27:740-745.
171. Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 2007;25:4722-4729.
172. Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer 2000;83:588-593.
173. Stupp R, Taillibert S, Kanner AA, et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015;314:2535-2543.
174. Ferguson SD, Xiu J, Weathers SP, et al. GBM-associated mutations and altered protein expression are more common in young patients. Oncotarget 2016;7:69466-69478.
175. Batchelor TT, Betensky RA, Esposito JM, et al. Age-dependent prognostic effects of genetic alterations in glioblastoma. Clin Cancer Res 2004;10:228-233.
176. Vuorinen V, Hinkka S, Farkkila M, et al. Debulking or biopsy of malignant glioma in elderly people - a randomised study. Acta Neurochir (Wien) 2003;145:5-10.
177. Almenawer SA, Badhiwala JH, Alhazzani W, et al. Biopsy versus partial versus gross total resection in older patients with high-grade glioma: a systematic review and meta-analysis. Neuro-oncology 2015;17:868-881.
178. Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol 2007;25:1824-1831.
179. VanderWalde N, Jagsi R, Dotan E, et al. NCCN Guidelines Insights: Older Adult Oncology, Version 2.2016. J Natl Compr Canc Netw 2016;14:1357-1370.
180. Perry JR, Laperriere N, O'Callaghan CJ, et al. Short-Course Radiation plus Temozolomide in Elderly Patients with Glioblastoma. The New England journal of medicine 2017;376:1027-1037.
181. Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brainstem gliomas. J Clin Oncol 2006;24:1266-1272.
182. Rineer J, Schreiber D, Choi K, et al. Characterization and outcomes of infratentorial malignant glioma: a population-based study using the Surveillance Epidemiology and End-Results database. Radiother Oncol 2010;95:321-326.
183. Recinos PF, Sciubba DM, Jallo GI. Brainstem tumors: where are we today? Pediatr Neurosurg 2007;43:192-201.
184. Bechet D, Gielen GG, Korshunov A, et al. Specific detection of methionine 27 mutation in histone 3 variants (H3K27M) in fixed tissue from high-grade astrocytomas. Acta neuropathologica 2014;128:733-741.
185. Khuong-Quang DA, Buczkowicz P, Rakopoulos P, et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta neuropathologica 2012;124:439-447.
186. Schwartzentruber J, Korshunov A, Liu XY, et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 2012;482:226-231.
187. Cavalheiro S, Yagmurlu K, da Costa MD, et al. Surgical approaches for brainstem tumors in pediatric patients. Childs Nerv Syst 2015;31:1815-1840.
188. Puget S, Beccaria K, Blauwblomme T, et al. Biopsy in a series of 130 pediatric diffuse intrinsic Pontine gliomas. Childs Nerv Syst 2015;31:1773-1780.
189. Carai A, Mastronuzzi A, De Benedictis A, et al. Robot-Assisted Stereotactic Biopsy of Diffuse Intrinsic Pontine Glioma: A Single-Center Experience. World neurosurgery 2017;101:584-588.
190. Hamisch C, Kickingereder P, Fischer M, et al. Update on the diagnostic value and safety of stereotactic biopsy for pediatric brainstem tumors: a systematic review and meta-analysis of 735 cases. J Neurosurg Pediatr 2017;20:261-268.
191. Cohen KJ, Heideman RL, Zhou T, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children's Oncology Group. Neuro-oncology 2011;13:410-416.
192. Sirachainan N, Pakakasama S, Visudithbhan A, et al. Concurrent radiotherapy with temozolomide followed by adjuvant temozolomide and cis-retinoic acid in children with diffuse intrinsic pontine glioma. Neuro-oncology 2008;10:577-582.
193. Gehring K, Sitskoorn MM, Gundy CM, et al. Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol 2009;27:3712-3722.
194. Khan F, Amatya B, Ng L, et al. Multidisciplinary rehabilitation after primary brain tumour treatment. Cochrane Database Syst Rev 2013:CD009509.
195. Poggi G, Liscio M, Pastore V, et al. Psychological intervention in young brain tumor survivors: the efficacy of the cognitive behavioural approach. Disabil Rehabil 2009;31:1066-1073.
196. Brem S S BPJ, Black P, et al. NCCN Clinical Practice Guidelines in Oncology: Central Nervous System Cancers. Journal of the National Comprehensive Cancer Network 2014;3:644-690.
197. 中国医师协会神经外科医师分会脑胶质瘤专业委员会. 胶质瘤多学科诊治(MDT)中国专家共识. 中华神经外科杂志 2018;34:113-118.
附录一:证据等级(牛津循证医学中心2011版)
| (临床)问题 | 步骤1 | 步骤2 | 步骤3 | 步骤4 | 步骤5 |
| (等级1*) | (等级2*) | (等级3*) | (等级4*) | (等级5*) | |
| 这个疾病有多普遍?(患病率) | 当地的,当前的随机样本调查(或普查) | 与当地情况相匹配调查的系统综述** | 当地的,非随机样本调查** | 病例系列** | N/A |
| 诊断或监测试验是否准确(诊断) | 一致地应用了参考标准和盲法的横断面研究的系统综述 | 一致地应用了参考标准和盲法的横断面研究 | 非连续病例研究,或研究未能一致地应用参考标准** | 病例对照研究,或应用了差的或非独立的参考标准** | 基于机制的推理 |
| 若不加这个治疗会发生什么?(预后) | 起始队列研究的系统综述 | 起始队列研究 | 队列研究或随机研究的对照组** | 病例系列或病例对照研究,或低质量预后队列研究** | N/A |
| 这个治疗有用吗? (治疗效益) | 随机试验或单病例随机对照试验的系统综述 | 随机试验或具有巨大效果的观察性研究 | 非随机对照队列/随访研究** | 病例系列,病例对照研究,或历史对照研究** | 基于机制的推理 |
| 这个治疗常见的伤害是什么(治疗伤害) | 随机试验的系统综述,巢式病例对照研究的系统综述,针对你所提临床问题患者的n-of-1试验,具有巨大效果的观察性研究 | 单个随机试验或(特殊地)具有巨大效果的观察性研究 | 非随机对照队列/随访研究(上市后监测)提供,足够数量来排除常见的伤害(对长期伤害需要足够长的随访时间)** | 病例系列,病例对照研究,或历史对照研究** | 基于机制的推理 |
| 这个治疗少见的伤害是什么?(治疗伤害) | 随机试验或N-of-1试验的系统综述 | 随机试验或(特殊地)具有巨大效果的观察性研究 | |||
| 这个试验(早期发现)值得吗? (筛查) | 随机研究的系统综述 | 随机试验 | 非随机对照队列/随访研究** | 病例系列,病例对照研究,或历史对照研究** | 基于机制的推理 |
* 根据研究质量,精确度,间接性,各个研究间不一致,或绝对效应值小,证据等级会被调低;若效应值很大,等级会被上调
**系统综述普遍地优于单个研究
附录二:脑胶质瘤分子诊断流程
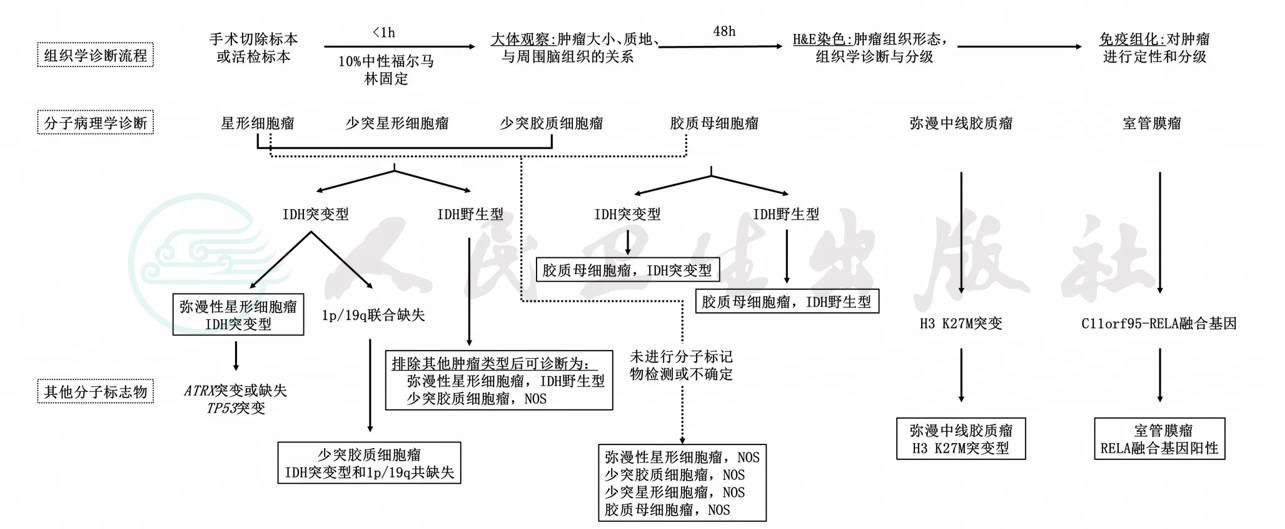
附录三:脑胶质瘤治疗流程
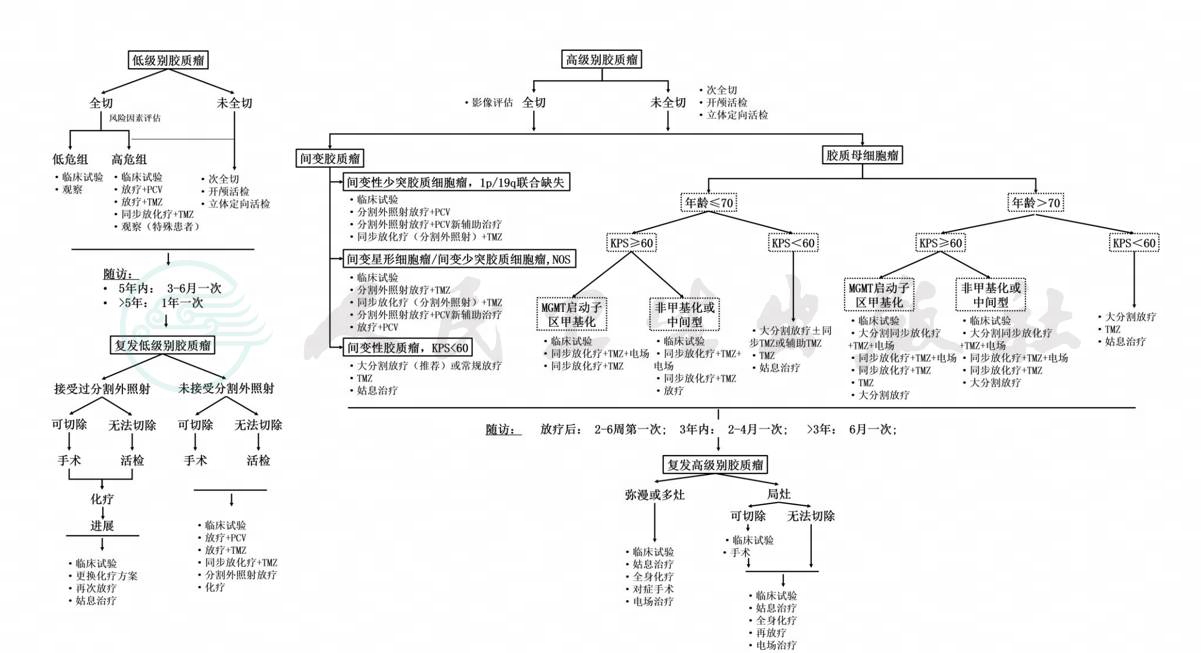
附录四:多学科诊疗模式(MDT)标准化流程
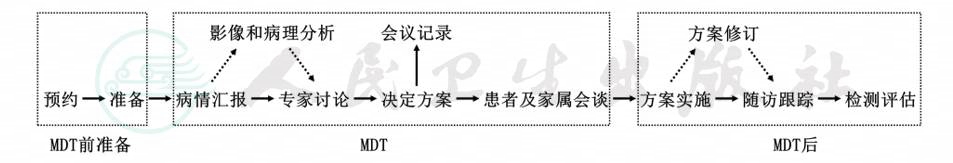
*实线箭头表示必须进行的过程;虚线箭头表示视情况而定,根据不同病例的特点决定是否进行。