 去看看
去看看


1 治疗前评估
【注释】
边缘区淋巴瘤(marginal zone lymphoma,MZL) 是一组B 细胞淋巴瘤,起源于淋巴滤泡的边缘区,可以发生于脾脏、淋巴结和黏膜淋巴组织。MZL 包括3 种类型,分别是黏膜相关淋巴组织(mucosa-associated lymphoid tissue,MALT) 结外MZL、结内MZL 和脾MZL。MZL 约占所有NHL 的10%,其中MALT 型结外MZL 所占比例最高,而原发胃的MZL 最为常见[1]。MZL 的病因与某些炎症抗原的慢性免疫刺激有关,比如幽门螺旋杆菌(helicobacter pylori,HP) 导致胃MALT 淋巴瘤,其他病原体包括鹦鹉热衣原体、博氏疏螺旋体和空肠弯曲杆菌等[2]。此外,HCV 也被发现和某些脾MZL 和非胃MZL 有关[3]。
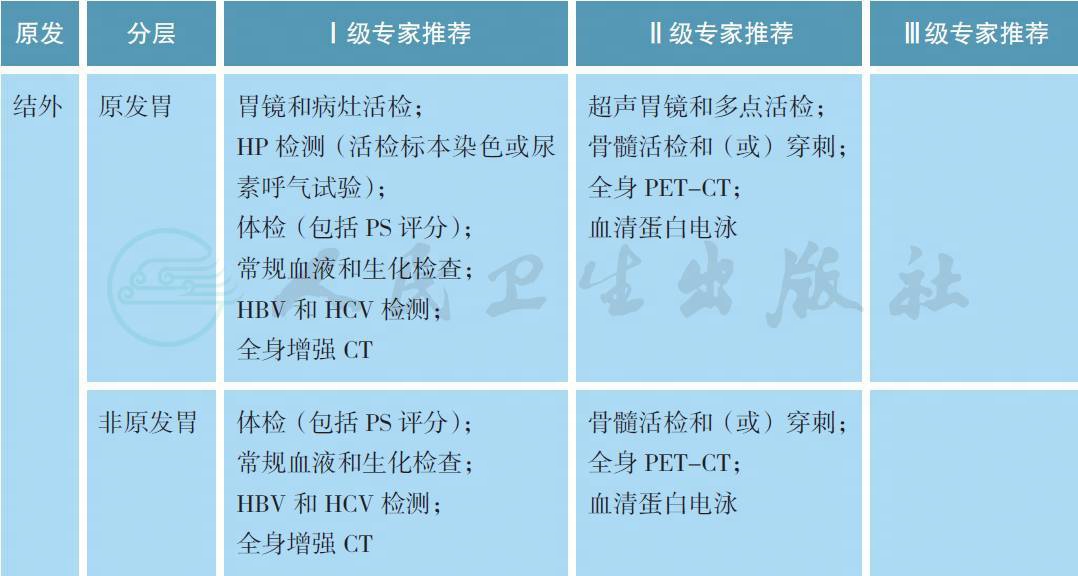
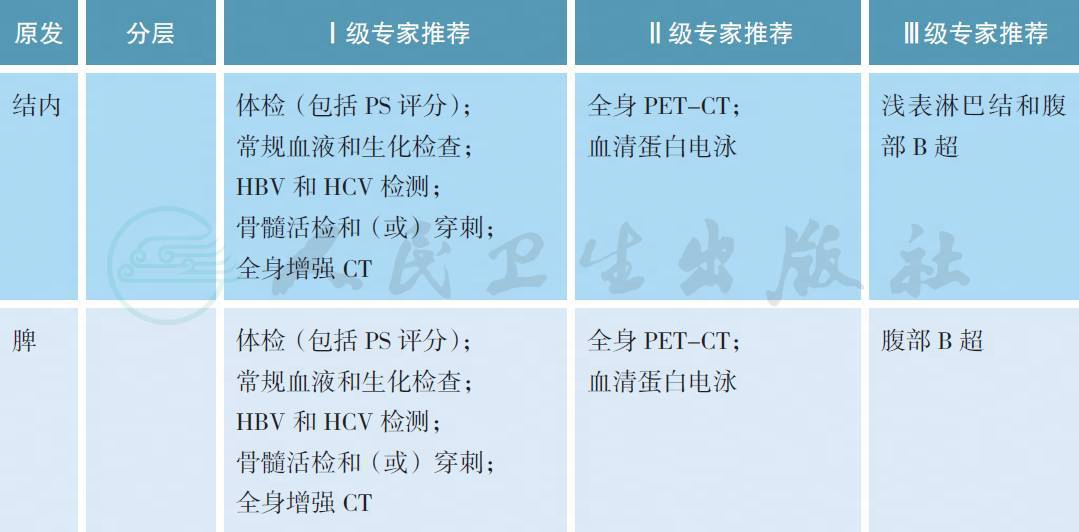
MZL 的治疗前评估除了淋巴瘤常规的体检、血液和生化检查、全身增强CT 以外,结内和脾MZL 需要接受骨髓活检和穿刺以明确分期,部分患者可以考虑进行全身PET-CT 检查。对于胃MZL,需要常规接受胃镜检查以及病灶部位的活检以明确病理以及HP 染色结果。欧洲胃肠淋巴瘤研究组推荐所有胃MZL 患者接受超声胃镜检查,有助于评价淋巴瘤浸润胃壁的深度,从而准确分期,同时进行多部位活检[4]。尿素呼气试验能够快速检测是否具有HP 感染,同时有助于重复评估抗HP的治疗效果。HCV 检测不但有助于部分MZL 的诊断,同时也可能作为治疗靶点。作为一种B 细胞淋巴瘤,利妥昔单抗可用于MZL 的治疗,因此HBV 检测也是常规的项目。
在预后因素方面,Ⅲ ~ Ⅳ期、年龄>70 岁和乳酸脱氢酶> 正常值上限是原发结外MALT 淋巴瘤3 个不利的预后因素,由此组成的MALT-IPI 将MALT 淋巴瘤分为低、中、高3 个危险分组,适用于原发胃和非原发胃的患者。
2 病理诊断
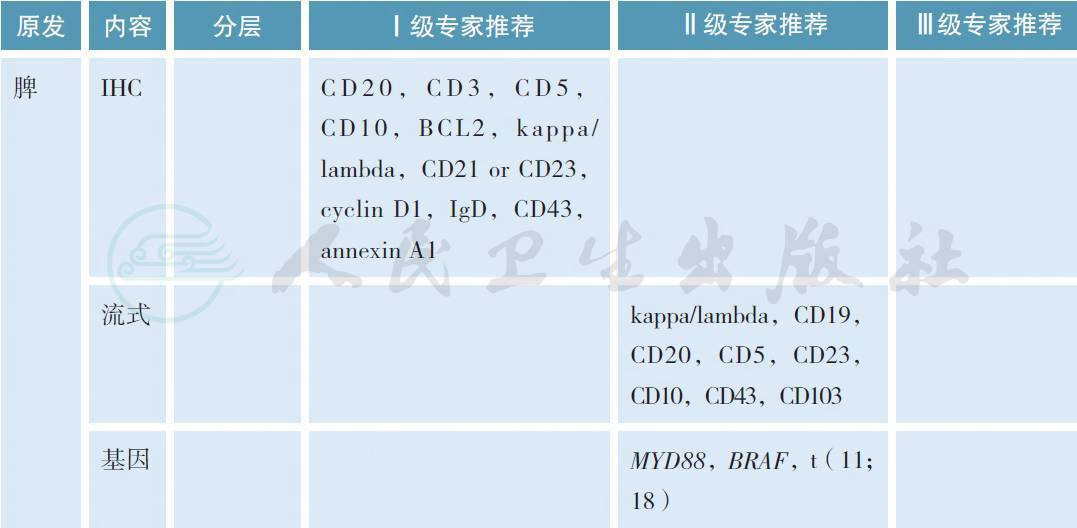
【注释】
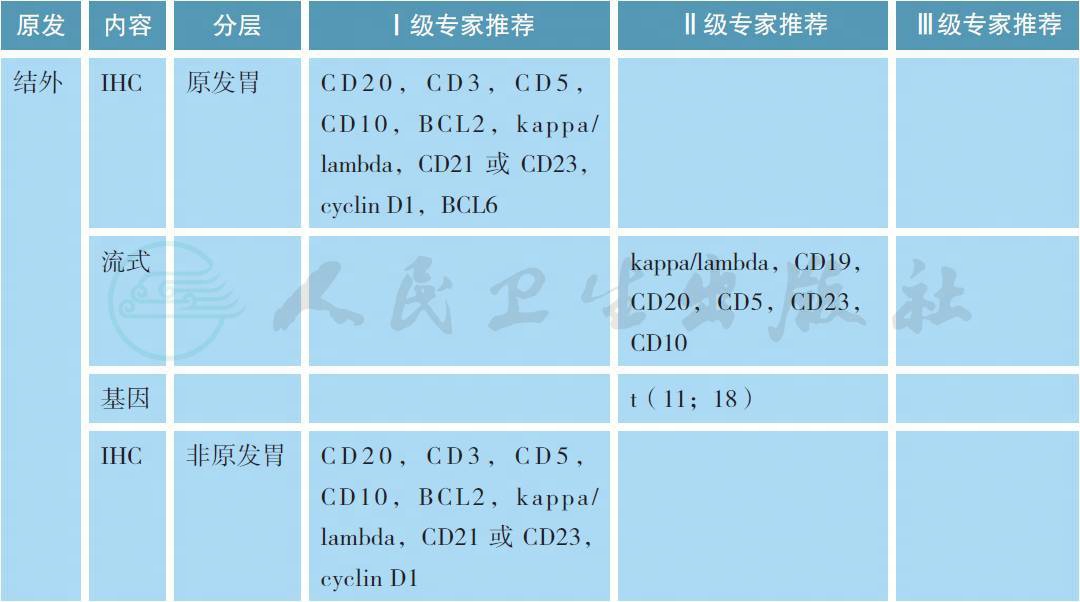
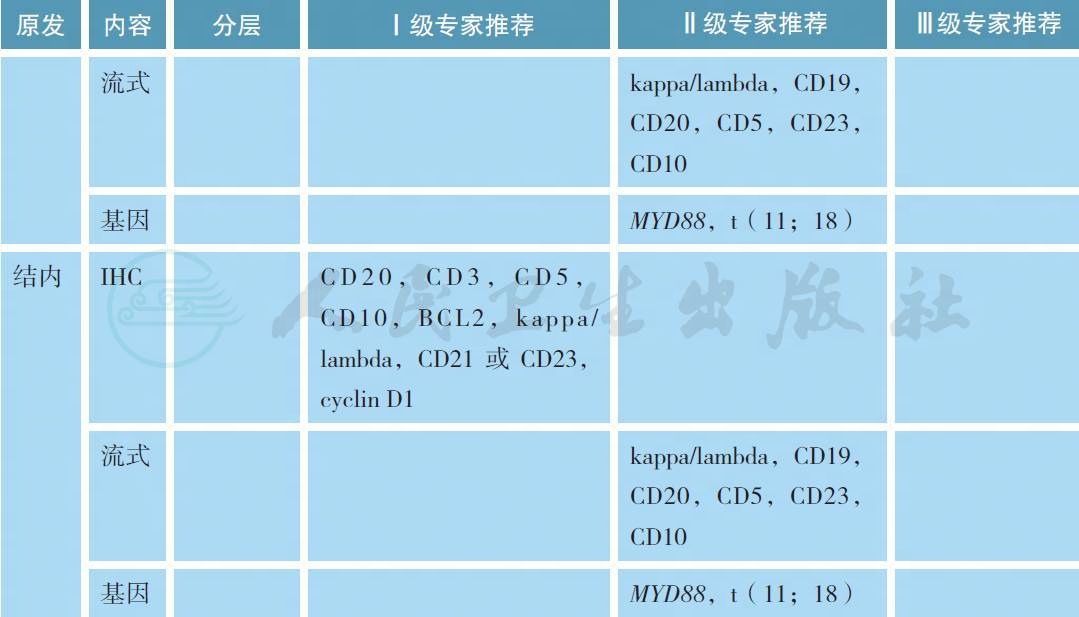
MZL 的病理学诊断应在有经验的病理实验室进行,标准应参照2016 版的WHO 淋巴肿瘤分类[6]。所有病理标本应常规进行免疫组织化学(IHC) 的检测,MZL 的典型免疫表型是CD5-,CD10-,CD20+,CD23-/+,CD43 -/+,cyclin D1- 和BCL2-,有条件的单位可以进行流式细胞的检测。部分MALT淋巴瘤可以出现(t 11;18),特别是HP阴性的胃MZL,常常预示疾病晚期和抗HP疗效欠佳
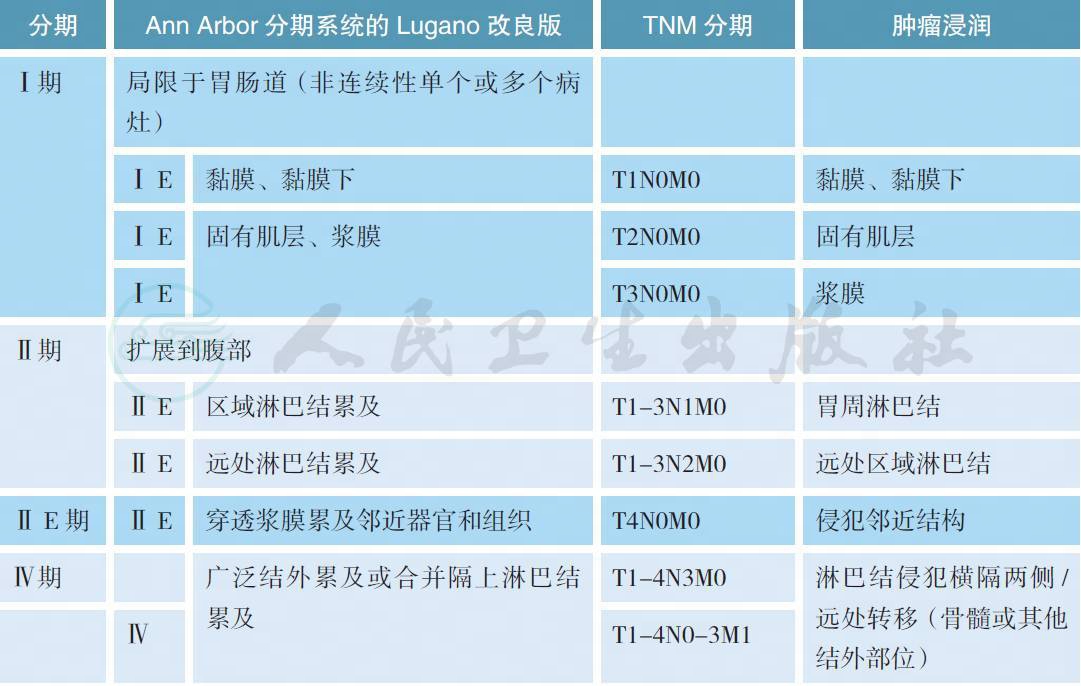
3 分期
目前淋巴瘤标准的分期系统是Lugano 分期,但对于MZL 通常适用于非胃或结内MZL[11]。胃肠MZL 通常采用Ann Arbor 分期系统的Lugano 改良版或胃肠淋巴瘤的TNM 分期(巴黎分期),而脾MZL 通常为脾脏单发,通过脾切除进行诊断和分期[12,13]。
4 治疗
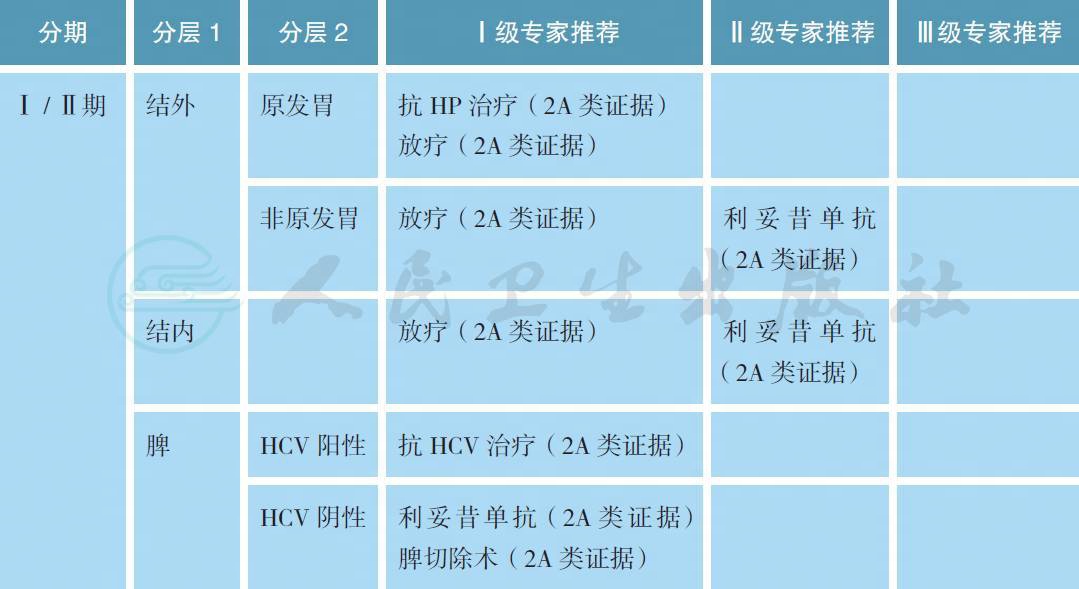
【注释】
MZL 的治疗策略应参考原发部位和疾病分期。对于局限期的MZL 患者,如果幽门螺旋杆菌(HP)阳性,强烈推荐抗HP 治疗[14-16]。抗HP 治疗后3 个月应复查HP 状态和胃镜,如果HP 转阴并且达到完全缓解(疗效评估采用GELA 标准[17]),则后续每6~12 个月复查胃镜直至5 年。如抗HP 治疗后肿瘤缓解或残留,如果肿瘤没有合并出血等症状,则后续每3~6 个月复查胃镜直至达到完全缓解。对于Ⅱ期、大包块和具有t(11;18) 的HP 阳性患者,研究表明抗HP 的疗效欠佳,如治疗后复查提示肿瘤缩小不明显应尽早给予放疗。对于HP 阴性的胃MZL,既往文献报道仍有一定比例的患者对于抗HP 治疗有效,这可能与假阴性或感染其他细菌所致,但治疗中需要密切观察以防短期内疾病进展[18]。对于抗HP 治疗后肿瘤持续残留或者合并出血等症状,放疗是常用的解救治疗模式[19,20]。其他结外MZL 也可能与些特定病原体感染有关,如眼附属器淋巴瘤与鹦鹉热衣原体有关,采用多西环素治疗具有很好的疗效[21-23]。此外,原发皮肤和小肠结外边缘区淋巴瘤分别与博氏疏螺旋体和空肠弯曲杆菌感染有关,但抗感染治疗的证据十分有限。总之,对于原发胃以外部位的Ⅰ / Ⅱ期结外MZL,放疗仍然是常用的治疗手段,部分不适合的患者可以考虑利妥昔单抗单药治疗。
对于Ⅰ / Ⅱ期结内MZL,放疗是常用的治疗手段,部分不适合的患者可以考虑利妥昔单抗单药治疗。大样本资料显示,首程未接受放疗患者有较高的淋巴瘤相关病死率,显著高于放疗病人[24]。对于脾MZL,脾切除术既是诊断也是治疗手段。对于未经脾切除术的MZL 患者,如果HCV 阳性,可以考虑行抗HCV 治疗[25]。如果HCV 阴性且患者具有脾肿大导致的血细胞下降或不适症状,利妥昔单抗单药是首选的治疗手段,而脾切除术可作为挽救治疗手段[26]。
放疗照射野采用受累部位照射(ISRT),不做预防照射,根据受侵器官,临床靶区(CTV) 通常需要包括整个器官,如眼、腮腺和全胃照射,放疗可以保存器官功能。根治性照射剂量24~30Gy,每次1.5~2.0Gy。姑息性放疗的照射剂量为2 × 2Gy 或其他剂量分割模式。
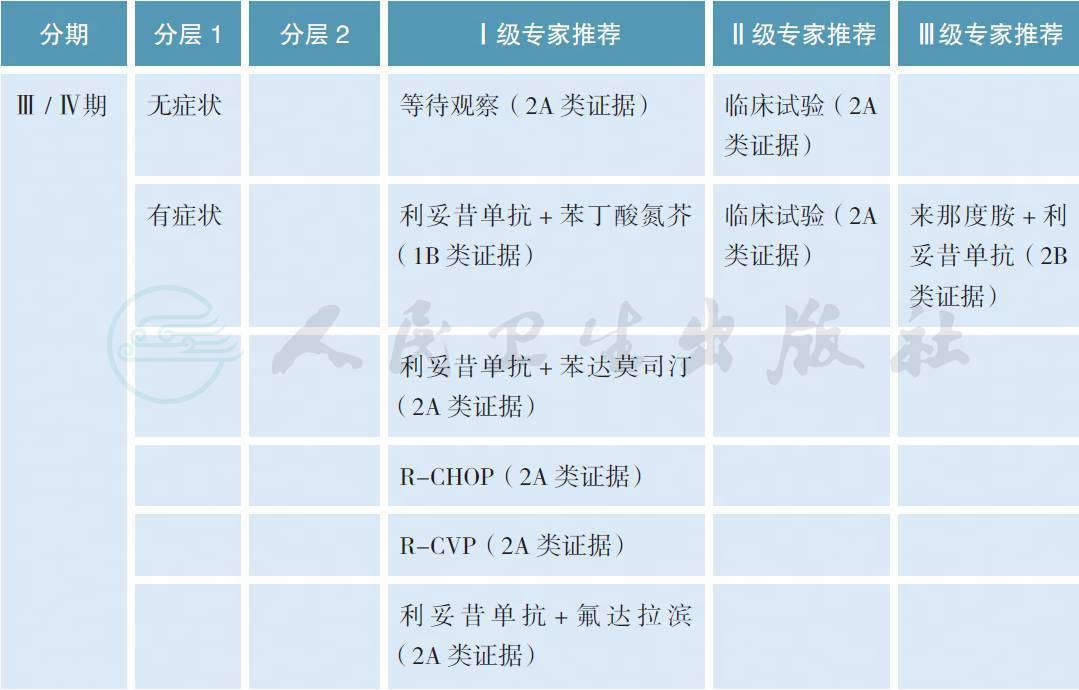
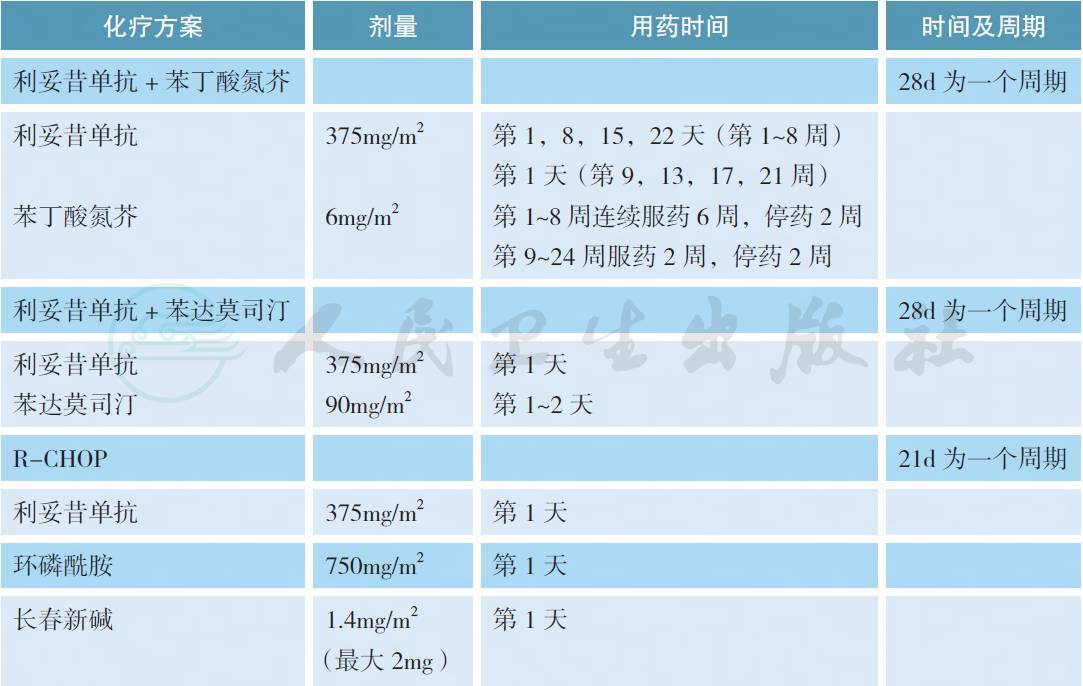
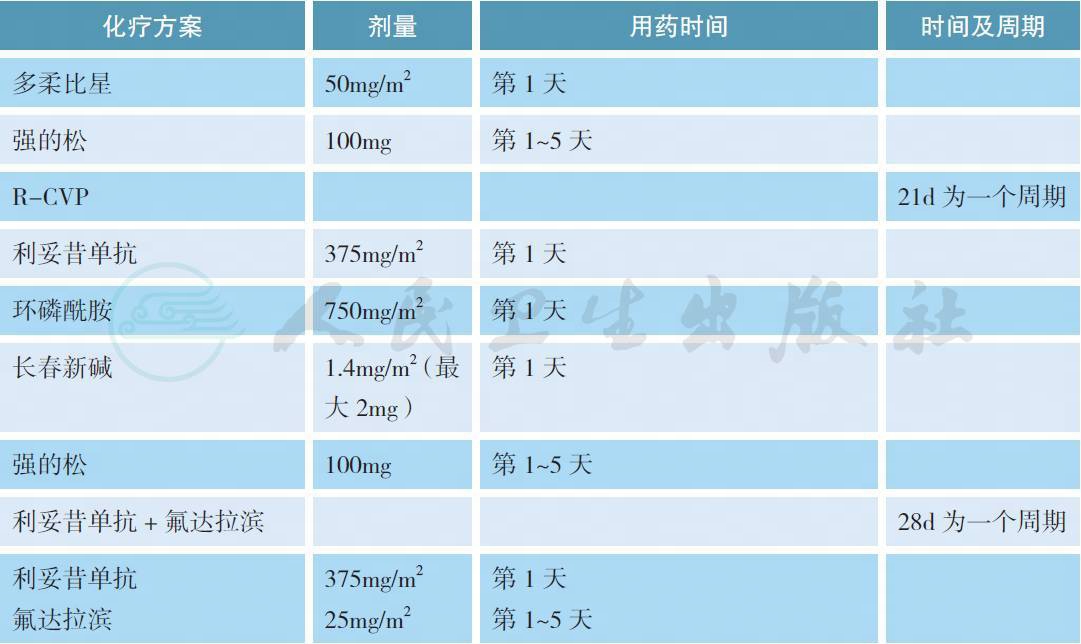
对于Ⅲ / Ⅳ期或者经局部放疗失败的边缘区淋巴瘤,如果没有B 症状、出血、血细胞下降、大包块或肿瘤快速进展等情况,可以参照惰性淋巴瘤的治疗原则给予等待观察。如果有上述情况,利妥昔单抗联合化疗是常用的治疗模式,但目前缺乏最佳的治疗方案。在一项名为IELSG-19 的Ⅲ期随机对照研究中,与单药苯丁酸氮芥和利妥昔单抗相比,利妥昔单抗联合苯丁酸氮芥获得较高的完全缓解率、无事件生存和无进展生存,但总生存3 组没有差别[27]。在另一项针对惰性淋巴瘤的Ⅲ期随机对照研究中,利妥昔单抗联合苯达莫司汀优于传统的R-CHOP 方案,但在MZL 的亚组分析中没有差别[28]。在其他单独针对MZL 的Ⅱ期临床研究中,利妥昔单抗分别联合苯达莫司汀、CHOP、CVP 和氟达拉滨也获得了很好的治疗效果[29-32]。对于以往经包含利妥昔单抗方案治疗失败的边缘区淋巴瘤,如果既往治疗有效且缓解期超过1 年可以考虑使用原方案治疗(蒽环类药物除外),否之可改用其他方案。在一项前瞻性Ⅱ期临床研究中,依布替尼单药针对这部分患者获得了一定的解救治疗效果,总体缓解率为48%,中位无进展生存为14.2 个月[33]。总体而言,鉴于Ⅲ / Ⅳ期边缘区淋巴瘤缺乏一类证据的治疗方案,推荐患者参加临床试验也是合理的选择。
表1 常用Ⅲ / Ⅳ期边缘区淋巴瘤的治疗方案
[1]A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin′slymphoma. The Non-Hodgkin′s Lymphoma Classification Project. Blood,1997,89(11):3909-3918.
[2]Ponzoni M,Ferreri AJ. Bacteria associated with marginal zone lymphomas. Best Pract Res ClinHaematol,2017,30(1-2):32-40.
[3]Armand M,Besson C,Hermine O,et al. Hepatitis C virus - Associated marginal zone lymphoma. BestPract Res Clin Haematol,2017,30(1-2):41-49.
[4]Ruskoné-Fourmestraux A,Fischbach W,Aleman BM,et al. EGILS consensus report. Gastricextranodal marginal zone B-cell lymphoma of MALT. Gut,2011,60(6):747-758.
[5]Thieblemont C,Cascione L,Conconi A,et al. A MALT lymphoma prognostic index. Blood,2017,130(12):1409-1417.
[6]Swerdlow SH,Campo E,Pileri SA,et al. The 2016 revision of the World Health Organizationclassification of lymphoid neoplasms. Blood,2016,127(20):2375-2390.
[7]Ye H,Liu H,Raderer M,et al. High incidence of t(11;18)(q21;q21) in Helicobacter pylorinegativegastric MALT lymphoma. Blood,2003,101(7):2547-2555.
[8]Liu H,Ye H,Ruskone-Fourmestraux A,et al. T(11;18) is a marker for all stage gastric MALTlymphomas that will not respond to H. pylori eradication.Gastroenterology,2002,122(5):1286-1294.
[9]Gachard N,Parrens M,Soubeyran I,et al. IGHV gene features and MYD88 L265P mutation separatethe three marginal zone lymphoma entities and Waldenstrom macroglobulinemia/lymphoplasmacyticlymphomas. Leukemia,2013,27(1):183-189.
[10]Arcaini L,Zibellini S,Boveri E,et al. The BRAF V600E mutation in hairy cell leukemia and othermature B-cell neoplasms. Blood,2012,119(1):188-191.
[11]Cheson BD,Fisher RI,Barrington SF,et al. Recommendations for initial evaluation,staging,andresponse assessment of Hodgkin and non-Hodgkin lymphoma:the Lugano classification. J Clin Oncol,2014,32(27):3059-3068.
[12]Rohatiner A,d′Amore F,Coiffier B,et al. Report on a workshop convened to discuss the pathologicaland staging classifications of gastrointestinal tract lymphoma. Ann Oncol,1994,5(5):397-400.
[13]Ruskone-Fourmestraux A,Dragosics B,Morgner A et al. Paris staging system for primarygastrointestinal lymphomas. Gut,2003,52(6):912-913.
[14]Stathis A,Chini C,Bertoni F,et al. Long-term outcome following Helicobacter pylori eradication in a retrospective study of 105 patients with localized gastric marginal zone B-cell lymphoma of MALT type.Ann Oncol,2009,20(6):1086-1093.
[15]Zullo A,Hassan C,Cristofari F,et al. Effects of Helicobacter pylori eradication on early stage gastricmucosa-associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol,2010,8(2):105-110.
[16]Nakamura S,Sugiyama T,Matsumoto T,et al. Long-term clinical outcome of gastric MALTlymphoma after eradication of Helicobacter pylori:a multicentre cohort follow-up study of 420 patientsin Japan. Gut,2012,61(4):507-513.
[17]Copie-Bergman C,Wotherspoon AC,Capella C,et al. Gela histological scoring system for posttreatmentbiopsies of patients with gastric MALT lymphoma is feasible and reliable in routine practice.Br J Haematol,2013,160:47-52.
[18]Zullo A,Hassan C,Andriani A,et al. Treatment of low-grade gastric MALT lymphoma unresponsiveto Helicobacter pylori therapy:a pooled-data analysis. Med Oncol,2010,27:291-295.
[19]Wirth A,Gospodarowicz M,Aleman BM,et al. Long-term outcome for gastric marginal zonelymphoma treated with radiotherapy:a retrospective,multi-centre,International ExtranodalLymphoma Study Group study. Ann Oncol,2013,24(5):1344-1351.
[20]Ruskoné-Fourmestraux A,Matysiak-Budnik T,Fabiani B,et al. Exclusive moderate-doseradiotherapy in gastric marginal zone B-cell MALT lymphoma:Results of a prospective study with along term follow-up. Radiother Oncol,2015,117(1):178-182.
[21]Ferreri AJ,Ponzoni M,Guidoboni M,et al. Bacteria-eradicating therapy with doxycycline in ocularadnexal MALT lymphoma:a multicenter prospective trial. J Natl Cancer Inst,2006,98(19):1375-1382.
[22]Ferreri AJ,Govi S,Pasini E,et al. Chlamydophila psittaci eradication with doxycycline as first-linetargeted therapy for ocular adnexae lymphoma:final results of an international phase Ⅱ trial. J ClinOncol,2012,30(24):2988-2994.
[23]Han JJ,Kim TM,Jeon YK,et al. Long term outcomes of first-line treatment with doxycycline in patients with previously untreated ocular adnexal marginal zone B lymphoma. Ann Hematol,2015,94(4):575-581.
[24]Olszewski AJ,Desai A. Radiation therapy administration and survival in stage Ⅰ / Ⅱ extranodalmarginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Int J Radiat Oncol Biol Phys,2014,88(3):642-649.
[25]Kelaidi C,Rollot F,Park S,Tulliez M,Christoforov B,Calmus Y,et al. Response to antiviraltreatment in hepatitis C virus associated marginal zone lymphomas. Leukemia,2004,18:1711-1716.
[26]Kalpadakis C,Pangalis GA,Angelopoulou MK,et al. Should rituximab replace splenectomy in themanagement of splenic marginal zone lymphoma? Best Pract Res Clin Haematol,2018,31(1):65-72.
[27]Zucca E,Conconi A,Martinelli G,et al. Final Results of the IELSG-19 Randomized Trial ofMucosa-Associated Lymphoid Tissue Lymphoma:Improved Event-Free and Progression-Free SurvivalWith Rituximab Plus Chlorambucil Versus Either Chlorambucil or Rituximab Monotherapy. J ClinOncol,2017,35(17):1905-1912.
[28]Rummel MJ,Niederle N,Maschmeyer G,et al. Bendamustine plus rituximab versus CHOP plusrituximab as first-line treatment for patients with indolent and mantle-cell lymphomas:an open-label,multicentre,randomised,phase 3 non-inferiority trial. Lancet,2013,381(9873):1203-1210.
[29]Salar A,Domingo-Domenech E’Panizo C,et al. First-line response-adapted treatment with thecombination of bendamustine and rituximab in patients with mucosa-associated lymphoid tissuelymphoma (MALT2008–01):a multicentre,single-arm,phase 2 trial. Lancet Hematol,2014,1:e104-e111.
[30]Raderer M,Wohrer S,Streubel B,et al. Activity of rituximab plus cyclophosphamide,doxorubicin/mitoxantrone,vincristine and prednisone in patients with relapsed MALT lymphoma. Oncology,2006,70:411-417.
[31]Kang HJ,Kim WS,Kim SJ,et al. Phase Ⅱ trial of rituximab plus CVP combination chemotherapy foradvanced stage marginal zone lymphoma as a first-line therapy:Consortium for Improving Survival ofLymphoma (CISL) study. Ann Hematol,2012,91:543-551.
[32]Brown JR,Friedberg JW,Feng Y,et al. A phase 2 study of concurrent fludarabine and rituximab for the treatment of marginal zone lymphomas. Br J Haematol,2009,145(6):741-748.
[33]Noy A,de Vos S,Thieblemont C,et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood,2017,129(16):2224-2232.