 去看看
去看看


第一部分 总论
1.MDT组织架构
1.1 召集人(首席专家)
一般由从事结直肠诊治的权威专家担任。
对MDT项目全权负责,主持并参与讨论,合理分配讨论时间,协调组织讨论,当意见不一致时,负责以投票制或者其他形式决定意见的形成,最终总结并形成个体化的专业意见。
审核医疗记录并签名负责。
如本人不能参与MDT会诊,需委托另外一位专家代为主持。
1.2 各科相对固定的专家
各专科一般应包括:结直肠科(胃肠外科)、肿瘤内科、放疗科、肝胆外科、介入科、影像科、内镜诊断科、病理科等;如有条件,也可包括:生物治疗科、超声诊断科、造口治疗师、心理治疗师等。一般根据医院情况设置,必要时可请相关专科参加。
专家一般应具有副高职称或高年资主治医师以上资格。
承诺按时定期参与MDT讨论,如自己不能参加,要指派另外一位相应专家代替参与。
负责MDT病例的提供,包括新发、复发及少见罕见、疑难病例,安排患者预约,准备资料,例如影像科专家提前阅片,病理科专家提前阅片或提供讨论需要的特殊检查项目等。
参与讨论的各科专家负责对患者进行相关的体格检查,对每个病例进行讨论,解答其他专家的问题,提出本专业领域的独立的观点,达成共识。
负责对自己预约提交讨论的患者作最终的解释并安排患者的下一步处理。
审核医疗记录,签名负责。
1.3 记录员
负责对MDT会诊全程记录,包括讨论专家的发言和最终建议。
打印最终讨论意见并提交专家签名。
负责统计MDT病例的临床资料。
如本人不能参加,需委托一名相关人员代替。
1.4 秘书(协调员)
应由结直肠癌相关学科高年资住院医师或低年资主治医师担任。
协助召集人进行MDT的全程操作,包括会诊前准备、会诊中协调、会诊后跟踪。
统一受理各专家推荐的患者预约,收集资料,按先后顺序或病情轻重安排讨论顺序。会诊前制作好患者表格(形式可以为公布栏、纸质表格、网络微信等)。
负责通知MDT成员会诊时间、地点、特殊安排、注意事项等。
负责协调各专家的出勤,打印出勤签到表格,督促每位到会人员签名。
负责保管、存档讨论记录和相关资料。
如本人不能参加,需委托一名相关人员代替。
1.5 医疗机构
提供指定的场所和相关的设施。
指定MDT专家成员的组成及IT后勤相关人员,颁发专家任命证书,并监督其参与。
建立考勤登记及相对应的奖惩制度。
负责建立及完善MDT操作流程,并保证其整体的实施。
保证患者的私隐及相关医疗记录不向外泄露。
根据学科具体情况,设立MDT临床研究基金,定期组织基金的申报,执行和监督。
2.MDT场地及设施的建议
2.1 会诊室
独立空间,足够宽敞,可容纳15~30人以上,照明设施完善,并有较好的通风系统。
2.2 圆桌台椅
可容纳15人左右圆桌以便于各科医师近距离讨论病情,如参加人数较多,可增加后排座椅。
2.3 实物投影仪(触屏式电子示教屏)
以便于影像学图片及病历资料放大投影到银幕上,供各科医师分析及讨论病情,有条件的医院最好配备触屏式电子示教屏,使分析内容更便捷直观。
2.4 内网
应配备医院内网(有线/无线),通过内网可以连接医院数据库,查询和调取患者的影像学、实验室等相关检查结果及病历内容。
2.5 微信群
有条件的情况下,可以建立一个微信群,包含参与MDT会诊的各科医师,成员应注明科室及全名,利于辨别身份。可及时传达MDT相关的重要通知,例如MDT会诊时间或地点临时改动通知、待讨论患者资料及会诊目的、知照影像及病理科医生提前阅片,以及拟提请MDT专家讨论的问题可发到微信群里,使会诊更具有时效性,提高会诊的效率。
2.6 电脑
配备至少两台电脑(台式或手提),和实物投影仪兼容。一台查询和调取患者的实验室相关检查结果及病历内容、并记录;一台专用调取影像学资料。
2.7 打印机
供打印会诊单及相关需要纸质保存的病历资料。
2.8 检查室(私密检查床)
有条件可配备独立检查室或会诊室有一张隔离的私密检查床,床底前方放置移动台阶,方便患者上下床;床旁配备常规检查车,车内应放置检查相关用品,如手套,液状石蜡,纸巾等;检查室内应配备洗手池和消毒液,以便于医师检查后清洁双手。
2.9 候诊室
有条件可配备独立的等候区或房间,与会诊室互不干扰。可建立叫号系统或叫号管理员,以便及时通知患者进行会诊。
2.10 会谈室
有条件可配备独立的会谈室提供一个私密的空间,便于医师把MDT会诊意见传达给患者及家属。会谈室内配备诊治相关的知情同意书及人体解剖示意图,保证医患双方整个交流过程的直观化及传达信息准确、充分,提高患者及家属依从性。
3.MDT操作程序的建议
3.1 预约
患者一部分通过专家、专科门诊预约,一部分通过友科或兄弟医院转诊。患者通常需要提前预约。患者MDT讨论前需要完成必要的实验室、影像学、内镜、病理检查,经由副高以上职称医师的审核,报请MDT秘书统一安排。秘书根据情况,控制当次MDT讨论的患者数量。
3.2 准备
MDT秘书提前将当次MDT讨论的名单,包括姓名、住院号、病历号等,通过邮件、微信或院内MDT信息系统发送到MDT的影像学医师、病理科医师处,由他们提前阅片,疑难病例组织影像科室和病理科室内部集体讨论。门诊患者的基本资料由门诊医生收集整理,并填写MDT讨论申请单。住院患者由管床医师收集资料,安排医师统一汇报当次MDT讨论病例,以电脑幻灯片的形式制作汇报材料。
3.3 病情汇报
原则上,门诊患者由轮值医生汇报病情,住院患者由管床医生汇报。汇报时除了汇报病史、治疗经过、检验和检查结果,还要说明患者的疗效期望、经济状况、依从性,提请MDT讨论的目的和理由。
3.4 影像分析
由影像科专家现场分析影像学资料,解答临床各科医师的疑问,提出进一步影像学检查的建议。
3.5 专家讨论
在MDT召集人的主持下,由相关专科的专家提出自己的诊断和治疗策略,明确治疗目标:治愈性、潜在治愈和姑息性治疗。阐述各种治疗手段对该患者的适应证、禁忌证、预期疗效、可能的并发症和风险。
3.6 决定方案
以NCCN指南、ESMO指南、我国《结直肠癌诊疗规范》(2010年)和《结直肠癌肝转移诊断和综合治疗指南》以及本共识为指导,结合患者的具体情况,综合上述医生的诊疗意见,由MDT召集人最终确定合理的个体化治疗方案,并交由相关的专科具体实施。
3.7 患者及家属会谈
由预约专家负责向患者和家属说明会诊的意见,解释他们的疑问,并告知他们进一步诊疗顺序和相关专科联系人的接诊时间或者联系方式。
3.8 讨论记录
记录员将讨论结论记录在《MDT意见表》上,最好是电子病历,打印后其中一份交给患者和家属,另一份交由秘书统一保管。
3.9 方案实施
具体诊断和治疗措施交由相应的MDT专科成员完成。
3.10 监测评估
由医院医务管理部门定期组织专家,抽查病历,了解MDT讨论执行情况,监督规范化治疗的实施。
3.11 方案修订
如果具体实施治疗方案的MDT成员发现疗效不满意、疾病进展等情况,需要及时反馈,再次提请MDT讨论,修正治疗方案。
3.12 随访跟踪
所有MDT决策的治疗方案实施完成后,召集人定期组织专人通过电话、信件、邮件的形式对患者进行随访。定期向MDT成员反馈治疗疗效和预后,不断提高诊治水平。
第二部分 肿瘤评估及治疗决策流程
多学科团队讨论过程中,大多数患者治疗决策可参考文末折页“结肠癌和上段直肠癌的MDT流程”与“中下段直肠癌肝转移的MDT流程”所示原则进行。
1.影像学评估
1.1 原发灶的影像学评估
1.1.1 直肠原发灶的影像学评估
直肠癌的推荐检查方法

1.1.1.1 直肠原发灶的MRI评估
直肠癌的MRI评估要点

续表

1.1.1.2 直肠原发灶的MRI报告模板
直肠肿瘤下缘至肛缘的距离:
下段(<5cm);
中段(5~10cm);
上段(>10cm)。
肿瘤与腹膜返折的关系:上方,骑跨,下方。
T分期:
T1:侵犯至黏膜下层。
T2:侵犯至肌层。
T3:穿透肌层至直肠周围组织。
a:浸润深度<5mm;
b:浸润深度5~10mm;
c:浸润深度>10mm。
T4:
a:腹膜返折受累;
b:累及/未累及邻近器官。
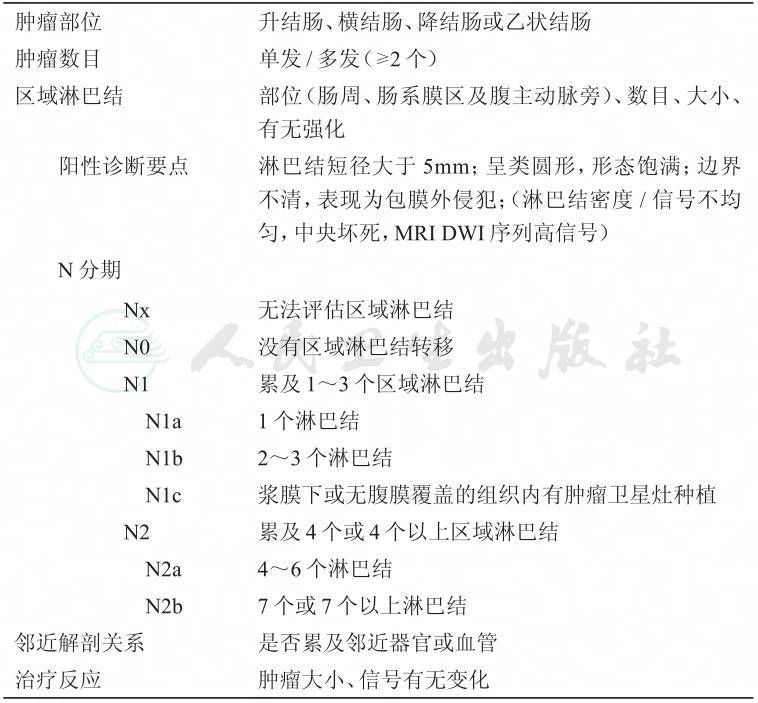
N分期:
直肠系膜见多个小淋巴结。
N0:未见转移性淋巴结。
N1:区域转移性淋巴结1~3个。
a:1个淋巴结;
b:2~3个淋巴结;
c:癌结节。
N2:区域转移性淋巴结≥4个。
a:4~6个淋巴结;
b:≥7个淋巴结。
盆壁淋巴结:阳性/阴性。
环周切缘(CRM):
环周切缘阳性,见于系列(Se)层面(Im),( )点钟。
环周切缘阴性。
肠壁外血管侵犯(EMVI):阳性,可疑,阴性。
(男性)前列腺及精囊腺大小、形态及信号未见明显异常。膀胱壁均匀、光整。盆腔未见积液。双侧腹股沟未见肿大淋巴结。
(女性)子宫及宫颈形态、大小正常,未见明显异常信号。双侧附件区未见明显异常信号。膀胱充盈良好,境界清楚,未见明显异常信号及强化灶。盆腔未见积液。双侧腹股沟未见肿大淋巴结。
诊断:
直肠上中下段癌(TN),CRM,EMVI。
1.1.1.3 直肠原发灶的腔内超声评估
直肠腔内超声是直肠癌局部分期应用较成熟的检查方法,研究表明它能准确判断肿瘤的侵犯深度,为术前分期及放化疗后疗效评价提供较可靠的依据。
直肠癌腔内超声评估要点:
对应直肠解剖学的五层结构,直肠腔内超声能清楚显示出五层超声分层及周围组织,表现为交替的高回声和低回声。
l 黏膜层(高回声)
l 黏膜肌层(低回声)
l 黏膜下层(高回声)
l 固有肌层(低回声)
l 浆膜层/肠周脂肪(高回声)
直肠腔内超声的分期主要针对TNM分期中的T分期,附上前缀“u”表明该分期是超声分期。低回声的肿瘤与超声分层的关系描述如下:
l uT0:肿瘤仅局限于黏膜层。
l uT1:肿瘤局限于黏膜层及黏膜下层。
l uT2:肿瘤侵犯固有肌层,但局限在肠壁内。
l uT3:肿瘤侵犯肠周组织,但未侵犯邻近器官。
l uT4:肿瘤侵犯邻近器官。
1.1.1.4 直肠原发灶的腔内超声报告模板
超声描述:
入肛××cm可探及肿物,病变最深位于××壁,深度约为××mm,范围约为××mm,内部回声不均匀,病灶处直肠黏膜层/黏膜下层回声模糊或者中断,固有肌层不均匀增厚,外膜回声连续/模糊/中断。病灶与周围组织分界清楚/不清。膀胱未见侵犯改变。(男性)前列腺、精囊腺未见侵犯改变。(女性)子宫、双附件区、阴道未见侵犯改变。
CDFI:上述病灶内可探及短棒状血流信号,测得动脉频谱Vp=××cm/s,RI=××。
肠旁/盆腔可探及×个淋巴结回声,大小/最大约为××mm,类圆形,淋巴门未能探及。
超声诊断:
直肠××壁实性肿物,考虑直肠癌,侵犯××层。肠旁淋巴结回声。
1.1.2 结肠原发灶的影像学评估
结肠癌的推荐检查方法

1.1.2.1 结肠原发灶的CT评估
结肠癌的CT评估要点

续表
1.1.2.2 结肠原发灶的CT报告模板
(升/横/降/乙状)结肠肠壁增厚,以(前、后、左、右)壁为主,最大层面大小约为____mm×mm,纵径约为____mm,病变环绕肠管(<1/4、1/4~1/2、1/2~3/4、3/4~全周)周径,平扫呈密度,增强扫描呈不均匀明显强化,局部管腔狭窄。病变处结肠浆膜面光整/欠光整,周围脂肪间隙清晰/模糊,其内可见条索影。肠周及肠系膜区(腹主动脉旁)见多个增大淋巴结影,较大者短径约为____mm。余各段结肠及直肠未见明显异常。
肝脏形态正常,各叶比例在正常范围内,外形轮廓光整,其内未见异常密度灶,增强扫描未见异常强化影。肝内胆管形态、走行正常,其内未见结石影。胆囊大小正常,胆总管未见扩张,其内均未见结石影。肝门区正常。门静脉形态正常,其内未见充盈缺损影。脾脏、胰腺大小、形态正常,密度均匀。双肾及双侧肾上腺大小、形态正常,未见异常密度影。
(男性)前列腺及精囊腺大小、形态及密度未见明显。膀胱壁均匀、光整。盆腔未见积液。
(女性)子宫及宫颈形态、大小正常,未见明显异常密度影。双侧附件区未见明显异常。膀胱充盈良好,境界清楚,未见明显异常密度影及强化灶。盆腔未见积液。
诊断结论:(升/横/降/乙状)结肠病灶,考虑结肠癌,(侵犯周围脂肪间隙),伴/不伴淋巴结转移。
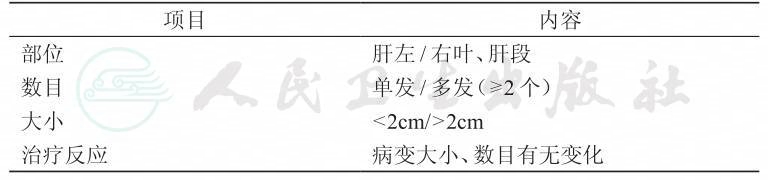
1.2 肝转移瘤的影像学评估
肝转移灶的推荐检查方法

*条件允许时,可行上腹部钆塞酸二钠(普美显)MRI平扫+增强检查以提高肝内小病灶的检出率
1.2.1 肝转移瘤的MRI评估
MRI是评价肝转移瘤的最重要手段,对于定性、定位及评估是否可切除起决定性作用。
肝转移灶的MRI评估要点

续表

1.2.2 肝转移瘤的MRI报告模板
肝脏形态正常,各叶比例在正常范围以内,其外形轮廓光整,密度/信号欠均匀,若病灶数目小于等于10个,则于肝Sn(n=1~8)见 个异常密度/信号病灶,大小分别为____cm,若病灶数目大于10个,则肝左叶/右叶/全肝见弥漫多个结节、肿块影,最大者位于肝Sn(Se,Im层面),大小为____,病灶平扫呈密度/T1 T2信号,边界不清,增强扫描动脉期、门脉期呈不均匀或环形强化(典型改变如“牛眼”征),病灶有/无侵犯肝左/中/右静脉、门静脉左支/右前支/右后支、下腔静脉或左/右肝管。
肝内胆管正常,胆囊大小正常,胆总管未见扩张;肝门区正常;门静脉所见正常;脾大小正常,密度/信号均匀;胰腺大小、形态正常,密度/信号均匀;双肾所见正常;双肾上腺所见正常。膈脚后/胃左/腹腔干/腹主动脉旁见肿大淋巴结,较大者短径约为____cm。
诊断结论:肝内单/多发病变,考虑转移瘤,有/无侵犯肝静脉、门静脉分支及下腔静脉和肝内胆管。
1.2.3 肝转移瘤的超声评估
肝转移灶的超声评估要点
1.2.4 肝转移瘤的超声报告模板
常规超声检查:
肝脏大小形态正常/失常,包膜光滑,实质回声均匀/不均匀,肝左、右叶/S×见单个、多个低回声/高回声病灶,大小约××mm,回声均匀/不均匀,边界清楚,周边可见低回声晕环。肝内血管走行正常。肝内胆管未见异常扩张。
CDFI:上述病灶内未见异常彩色血流信号。/可见点状血流信号,测得动脉频谱Vp=××cm/s,RI=××。
胆囊大小形态正常,囊壁薄光滑,腔内未见异常回声。
脾脏大小形态正常,实质回声均匀,未见明确占位性病变。
胰腺大小形态正常,实质回声均匀,未见明确占位性病变。胰管未见扩张。
超声造影检查:
经肘静脉弹丸注射SonoVue 2.4ml,肝S×病
1.Andreou A,Aloia TA,Brouquet A,et al.Margin status remains an important determinant of survival after surgical resection of colorectal liver metastases in the era of modern chemotherapy.Ann Surg,2013,257: 1079-1088.
2.Angelsen JH,Horn A,Eide GE,et al.Surgery for colorectal liver metastases: the impact of resection margins on recurrence and overall survival.World J Surg Oncol,2014,12: 127.
3.Are C,Gonen M,Zazzali K et al.The impact of margins on outcome after hepatic resection for colorectal metastasis.Ann Surg,2007,246: 295-300.
4.Ayez N,Lalmahomed ZS,Eggermont AM,et al.Outcome of microscopic incomplete resection (R1)of colorectal liver metastases in the era of neoadjuvant chemotherapy.Ann Surg Oncol,2012,19: 1618-1627.
5.de Haas RJ,Wicherts DA,Flores E,et al.R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery?Ann Surg,2008,248: 626-637.
6.Khan MA,Hakeem AR,Scott N,et al..Significance of R1 resection margin in colon cancer resections in the modern era.Colorectal Dis,2015,17: 943-953.
7.Malik H,Khan AZ,Berry DP,et al.Liver resection rate following downsizing chemotherapy with cetuximab in metastatic colorectal cancer: UK retrospective observational study.Eur J Surg Oncol,2015,41: 499-505.
8.Araujo R,Gonen M,Allen P,et al.Comparison between perioperative and postoperative chemotherapy after potentially curative hepatic resection for metastatic colorectal cancer.Ann Surg Oncol,2013,20: 4312-4321.
9.Bilchik AJ,Poston G,Adam R,et al..Prognostic variables for resection of colorectal cancer hepatic metastases: an evolving paradigm.J Clin Oncol,2008,26: 5320-5321.
10.Nordlinger B,Sorbye H,Glimelius B,et al.Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial.Lancet,2008,371: 1007-1016.
11.van Vledder MG,de Jong MC,Pawlik TM,et al.Disappearing colorectal liver metastases after chemotherapy: should we be concerned?.J Gastrointest Surg,2010,14: 1691-1700.
12.Benoist S,Brouquet A,Penna C et al.Complete response of colorectal liver metastases after chemotherapy: does it mean cure?.J Clin Oncol,2006,24: 3939-3945.
13.Bilchik AJ,Poston G,Curley SA,et al.Neoadjuvant chemotherapy for metastatic colon cancer: a cautionary note.J Clin Oncol,2005,23: 9073-9078.
14.Rubbia-Brandt L,Audard V,Sartoretti P,et al.Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer.Ann Oncol,2004,15: 460-466.
15.Vauthey JN,Pawlik TM,Ribero,D et al.Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases.J Clin Oncol,2006,24: 2065-2072.
16.Schmoll HJ,Cartwright T,Tabernero J,et al.PhaseⅢtrial of capecitabine plus oxaliplatin as adjuvant therapy for stageⅢcolon cancer: a planned safety analysis in 1,864 patients.J Clin Oncol,2007,25: 102-109.
17.Primrose J,Falk S,Finch-Jones M,et al.Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial.Lancet Oncol,2014,15: 601-611.
18.Sarpel U,Bonavia AS,Grucela A,et al.Does anatomic versus nonanatomic resection affect recurrence and survival in patients undergoing surgery for colorectal liver metastasis?Ann Surg Oncol,2009,16: 379-384.
19.Kulik U,Bektas H,Klempnauer J,et al..Repeat liver resection for colorectal metastases.Br J Surg,2013,100: 926-932.
20.Hamady ZZ,Lodge JP,Welsh FK,et al.One-millimeter cancer-free margin is curative for colorectal liver metastases: a propensity score case-match approach.Ann Surg,2014,259:543-548.
21.Yin Z,Liu C,Chen Y,et al.Timing of hepatectomy in resectable synchronous colorectal liver metastases (SCRLM): Simultaneous or delayed?Hepatology,2013,57: 2346-2357.
22.Brouquet A,Mortenson MM,Vauthey JN,et al.Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic,combined or reverse strategy?J Am Coll Surg,2010,210: 934-941.
23.Lam VW,Laurence JM,Pang T,et al.A systematic review of a liver‐first approach in patients with colorectal cancer and synchronous colorectal liver metastases.HPB,2014,16:101-108.
24.Embun R,Fiorentino F,Treasure T,et al.Pulmonary metastasectomy in colorectal cancer:a prospective study of demography and clinical characteristics of 543 patients in the Spanish colorectal metastasectomy registry (GECMP-CCR).BMJ Open,2013,3(5).pii: e002787.
25.Adam R,Delvart V,Pascal G,et al.Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival.Ann Surg,2004,240: 644-657;discussion 657-648.
26.Kishi Y,Zorzi D,Contreras CM et al.Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases.Ann Surg Oncol,2010,17: 2870-2876.
27.Jaeck D,Oussoultzoglou E,Rosso E,et al.A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases.Ann Surg,2004,240: 1037.
28.Abdalla EK,Adam R,Bilchik AJ,et al.Improving resectability of hepatic colorectal metastases: expert consensus statement.Ann Surg Oncol,2006,13: 1271-1280.
29.Hoekstra LT,van Lienden KP,Doets A,et al.Tumor progression after preoperative portal vein embolization.Ann Surg,2012,256: 812-817;discussion 817-818.
30.Zou RH,Li AH,Han F,et al.Liver hypertrophy and accelerated growth of implanted tumors in nonembolized liver of rabbit after left portal vein embolization.J Surg Res,2012,178: 255-263.
31.Schadde E,Ardiles V,Robles-Campos R,et al.Early survival and safety of ALPPS: first report of the International ALPPS Registry.Ann Surg,2014,260: 829-836;discussion 836-828.
32.Abdalla EK,Bauer TW,Chun YS,et al.Locoregional surgical and interventional therapies for advanced colorectal cancer liver metastases: expert consensus statements.HPB (Oxford),2013,15: 119-130.
33.Pawlik TM,Izzo F,Cohen DS,et al.Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients.Ann Surg Oncol,2003,10(9): 1059-1069.
34.Petrowsky H,Gonen M,Jarnagin W,et al.Second liver resections are safe and effective treatment for recurrent hepatic metastases from colorectal cancer: a bi-institutional analysis.Ann Surg,2002,235: 863-871.
35.Yan TD,Sim J,Black D et al.Systematic review on safety and efficacy of repeat hepatectomy for recurrent liver metastases from colorectal carcinoma.Ann Surg Oncol,2007,14: 2069-2077.
36.Meyerhardt JA,Mangu PB,Flynn PJ,et al.Follow-up care,surveillance protocol,and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement.J Clin Oncol,2013,31: 4465-4470.
37.Hamada A,Yamakado K,Nakatsuka A,et al.Radiofrequency ablation for colorectal liver metastases: prognostic factors in non-surgical candidates.Jpn J Radiol,2012,30: 567-574.
38.Hammill CW,Billingsley KG,Cassera MA,et al.Outcome after laparoscopic radiofrequency ablation of technically resectable colorectal liver metastases.Ann Surg Oncol,2011,18: 1947-1954.
39.Solbiati L,Ahmed M,Cova L,et al.Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up.Radiology,2012,265: 958-968.
40.Veltri A,Sacchetto P,Tosetti I,et al.Radiofrequency ablation of colorectal liver metastases:small size favorably predicts technique effectiveness and survival.Cardiovasc Intervent Radiol,2008,31: 948-956.
41.Kim KH,Yoon YS,Yu CS,et al.Comparative analysis of radiofrequency ablation and surgical resection for colorectal liver metastases.J Korean Surg Soc,2011,81: 25-34.
42.Ruers T,Punt C,Van Coevorden F,et al.Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phaseⅡstudy (EORTC 40004).Ann Oncol,2012,23: 2619-2626.
43.Kingham TP,Karkar AM,D’Angelica MI et al.Ablation of perivascular hepatic malignant tumors with irreversible electroporation.J Am Coll Surg 2012,215: 379-387.
44.Ogawa T,Kawamoto H,Kobayashi Y et al.Prevention of biliary complication in radiofrequency ablation for hepatocellular carcinoma-Cooling effect by endoscopic nasobiliary drainage tube.Eur J Radiol,2010,73: 385-390.
45.Silk MT,Wimmer T,Lee KS,et al.Percutaneous ablation of peribiliary tumors with irreversible electroporation.J Vasc Interv Radiol,2014,25: 112-118.
46.Alberts SR,Horvath WL,Sternfeld WC,et al.Oxaliplatin,fluorouracil,and leucovorin for patients with unresectable liver-only metastases from colorectal cancer: a North Central Cancer Treatment Group phaseⅡstudy.J Clin Oncol,2005,23: 9243-9249.
47.Pozzo C,Basso M,Cassano A,et al.Neoadjuvant treatment of unresectable liver disease with irinotecan and 5-fluorouracil plus folinic acid in colorectal cancer patients.Ann Oncol,2004,15: 933-939.
48.Delaunoit T,Alberts SR,Sargent DJ,et al.Chemotherapy permits resection of metastatic colorectal cancer: experience from Intergroup N9741.Ann Oncol,2005,16: 425-429.
49.Folprecht G,Gruenberger T,Bechstein WO,et al.Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial.Lancet Oncol,2010,11: 38-47.
50.Ye LC,Liu TS,Ren L,et al.Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases.J Clin Oncol,2013,31: 1931-1938.
51.Falcone A,Ricci S,Brunetti I,et al.PhaseⅢtrial of infusional fluorouracil,leucovorin,oxaliplatin,and irinotecan (FOLFOXIRI) compared with infusional fluorouracil,leucovorin,and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest.J Clin Oncol,2007,25: 1670-1676.
52.Souglakos J,Androulakis N,Syrigos K,et al.FOLFOXIRI (folinic acid,5-fluorouracil,oxaliplatin and irinotecan) vs FOLFIRI (folinic acid,5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a multicentre randomised phaseⅢtrial from the Hellenic Oncology Research Group (HORG).Br J Cancer,2006,94: 798-805.
53.Masi G,Vasile E,Loupakis F,et al.Randomized trial of two induction chemotherapy regimens in metastatic colorectal cancer: an updated analysis.J Natl Cancer Inst,2011,103: 21-30.
54.Fuchs CS,Marshall J,Mitchell E,et al.Randomized,controlled trial of irinotecan plus infusional,bolus,or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study.J Clin Oncol 2007,25: 4779-4786.
55.Simkens LH,van Tinteren H,May A,et al.Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group.Lancet,2015,385: 1843-1852.
56.Chibaudel B,Maindrault-Goebel F,Lledo G,et al.Can chemotherapy be discontinued in unresectable metastatic colorectal cancer?The GERCOR OPTIMOX2 Study.J Clin Oncol,2009,27: 5727-5733.
57.Andre T,Louvet C,Maindrault-Goebel F,et al.CPT-11 (irinotecan) addition to bimonthly,high-dose leucovorin and bolus and continuous-infusion 5-fluorouracil (FOLFIRI) for pretreated metastatic colorectal cancer.GERCOR.Eur J Cancer,1999,35: 1343-1347.
58.Hurwitz HI,Fehrenbacher L,Hainsworth JD,et al.Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer.J Clin Oncol,2005,23: 3502-3508.
59.Van Cutsem E,Hoff PM,Harper P,et al.Oral capecitabine vs intravenous 5-fluorouracil and leucovorin: integrated efficacy data and novel analyses from two large,randomised,phaseⅢtrials.Br J Cancer,2004,90: 1190-1197.
60.Van Cutsem E,Twelves C,Cassidy J,et al.Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phaseⅢstudy.J Clin Oncol,2001,19: 4097-4106.
61.Hochster HS,Hart LL,Ramanathan RK,et al.Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study.J Clin Oncol,2008,26: 3523-3529.
62.McCahill LE,Yothers G,Sharif S,et al.Primary mFOLFOX6 plus bevacizumab without resection of the primary tumor for patients presenting with surgically unresectable metastatic colon cancer and an intact asymptomatic colon cancer: definitive analysis of NSABP trial C-10.J Clin Oncol,2012,30: 3223-3228.
63.Poultsides GA,Servais EL,Saltz LB,et al.Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment.J Clin Oncol,2009,27: 3379-3384.
64.Nitzkorski JR,Farma JM,Watson JC,et al.Outcome and natural history of patients with stage IV colorectal cancer receiving chemotherapy without primary tumor resection.Ann Surg Oncol,2012,19: 379-383.
65.Tebbutt NC,Norman AR,Cunningham D,et al.Intestinal complications after chemotherapy for patients with unresected primary colorectal cancer and synchronous metastases.Gut,2003,52: 568-573.
66.Scheer MG,Sloots CE,van der Wilt GJ,et al.Management of patients with asymptomatic colorectal cancer and synchronous irresectable metastases.Ann Oncol 2008,19: 1829-1835.
67.Cirocchi R,Trastulli S,Abraha I,et al.Non-resection versus resection for an asymptomatic primary tumour in patients with unresectable stageⅣcolorectal cancer.Cochrane Database Syst Rev,2012,8: CD008997.
68.M.Faron,A.Bourredjem,J-P.Pignon,et al.Impact on survival of primary tumor resection in patients with colorectal cancer and unresectable metastasis: Pooled analysis of individual patients’data from four randomized trials.J Clin Oncol,30,2012 (suppl;abstr 3507).
69.Rahbari NN,Lordick F,Fink C,et al.Resection of the primary tumour versus no resection prior to systemic therapy in patients with colon cancer and synchronous unresectable metastases (UICC stageⅣ): SYNCHRONOUS—a randomised controlled multicentre trial(ISRCTN30964555).BMC Cancer,2012,12: 142.
70.Tarantion I,et al.Ann Surg.2014 Nov,4.
71.Hu CY Bailey CE,You YN,et al.Time Trend Analysis of Primary Tumor Resection for Stage IV Colorectal Cancer: Less Surgery,Improved Survival.JAMA Surg,2015,150(3):245-251.
72.Rahbari N,Lordick F,Fink C,et al.Resection of the primary tumour versus no resection prior to systemic therapy in patients with colon cancer and synchronous unresectable metastases (UICC stageⅣ): SYNCHRONOUS-a randomised controlled multicentre trial(ISRCTN30964555).BMC Cancer,2012,12:142.
73.Chen G,Zhang RX,Li YH,et al.Multi-center,randomized,controlled,open-label effectiveness study of primary tumor resection or not in asymptomatic colorectal cancer with unresectable metastatic disease.J Clin Oncol 33,2015 (suppl;abstr TPS 3628).
74.Adam R,de Gramont A,Figueras J,et al.Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus.Cancer Treat Rev,2015,41(9):729-741.
75.Hellman S,Weichselbaum RR.Oligometastases.J Clin Oncol,1995,13:8-10.