 去看看
去看看


一、概要
影像和分期诊断
病理学诊断
上述证据级别全部为2A类证据
分子分型
分子分型(续)
基于病理类型、分期和分子分型的综合治疗
非小细胞肺癌的治疗
ⅠA、ⅠB期原发性非小细胞肺癌的治疗

ⅡA、ⅡB期原发性非小细胞肺癌的治疗
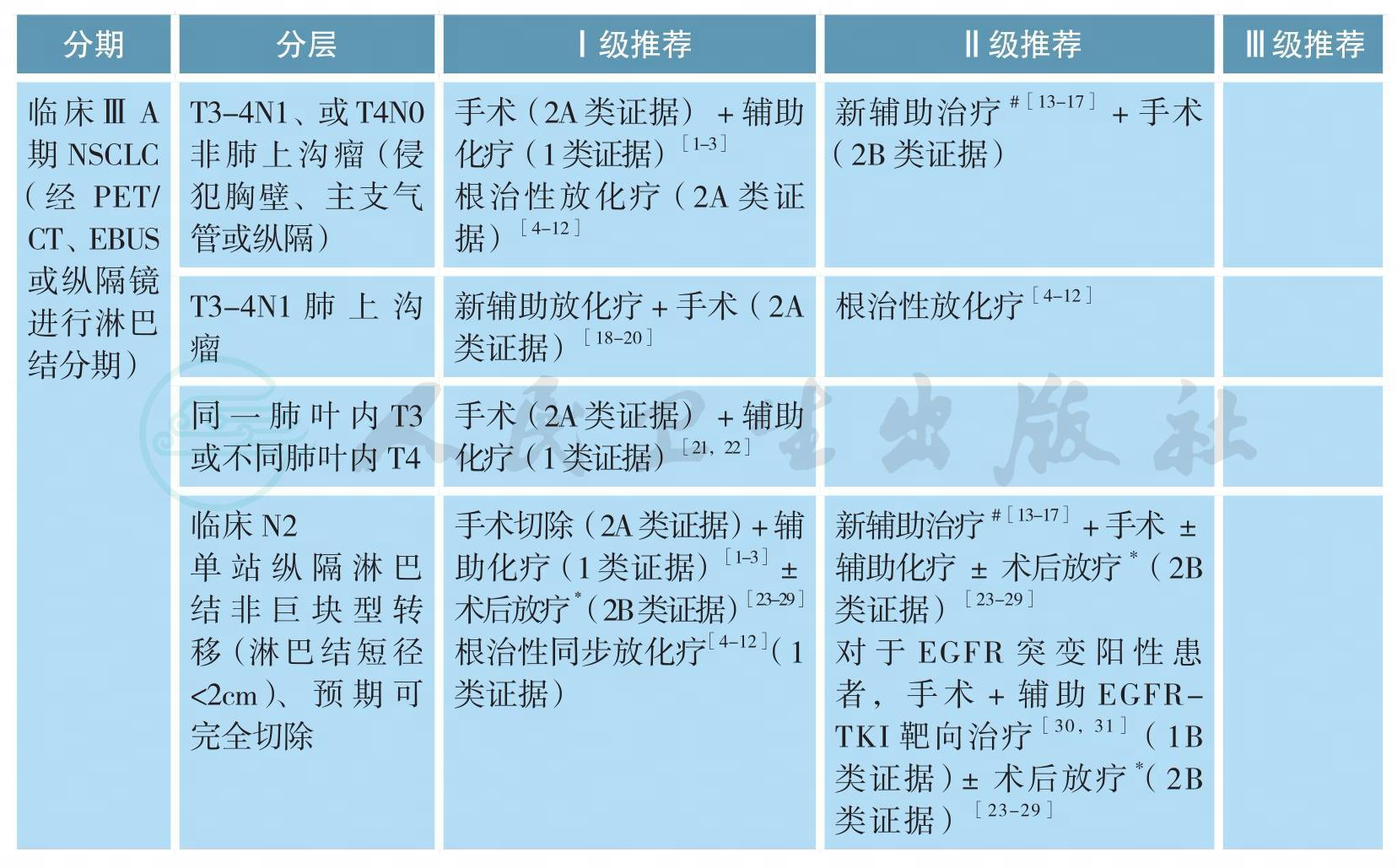
可手术ⅢA期原发性非小细胞肺癌的治疗
可手术ⅢA期原发性非小细胞肺癌的治疗(续)
# 新辅助治疗模式包括:单纯化疗、序贯化放疗、同步放化疗、化疗后同步放化疗等,最佳模式尚未确定
[13-17]*术后病理N2可以考虑术后放疗(2B类证据)或加入术后放疗随机分组研究
[23-29]**该组患者的局部区域复发风险较单站N2淋巴结转移患者进一步升高,术后病理N2可以考虑术后放疗(2B类证据)
[23-29]不可手术ⅢA、ⅢB期原发性非小细胞肺癌的治疗

不可手术ⅢA、ⅢB期原发性非小细胞肺癌的治疗(续)

备注:不可切除ⅢA期、ⅢB期主要指有如下影像或淋巴结病理性证据:
1.同侧纵隔淋巴结多枚转移成巨大肿块或多站转移(ⅢA:T1-3N2或ⅢB:T4N2)。
2.对侧肺门、纵隔淋巴结,或同、对侧斜角肌或锁骨上淋巴结转移(ⅢB:T1-4N3)。
3.病灶侵犯心脏、主动脉和食管(ⅢB:T4N0-1)。
同步放化疗方案:
EP:顺铂 50mg/m2,d1,8,29,36;依托泊苷 50mg/m2,d1~5,d29~30;
PC:卡铂AUC 2,紫杉醇45~50mg/m2,每周;
AP:顺铂75mg/m2,d1;培美曲塞500mg/m2,d1,每3周重复(非鳞癌);
AC:卡铂AUC 5,d1;培美曲塞500mg/m2,d1,每3周重复(非鳞癌)。
放疗方案:60~66Gy/30~33次/6~7周。
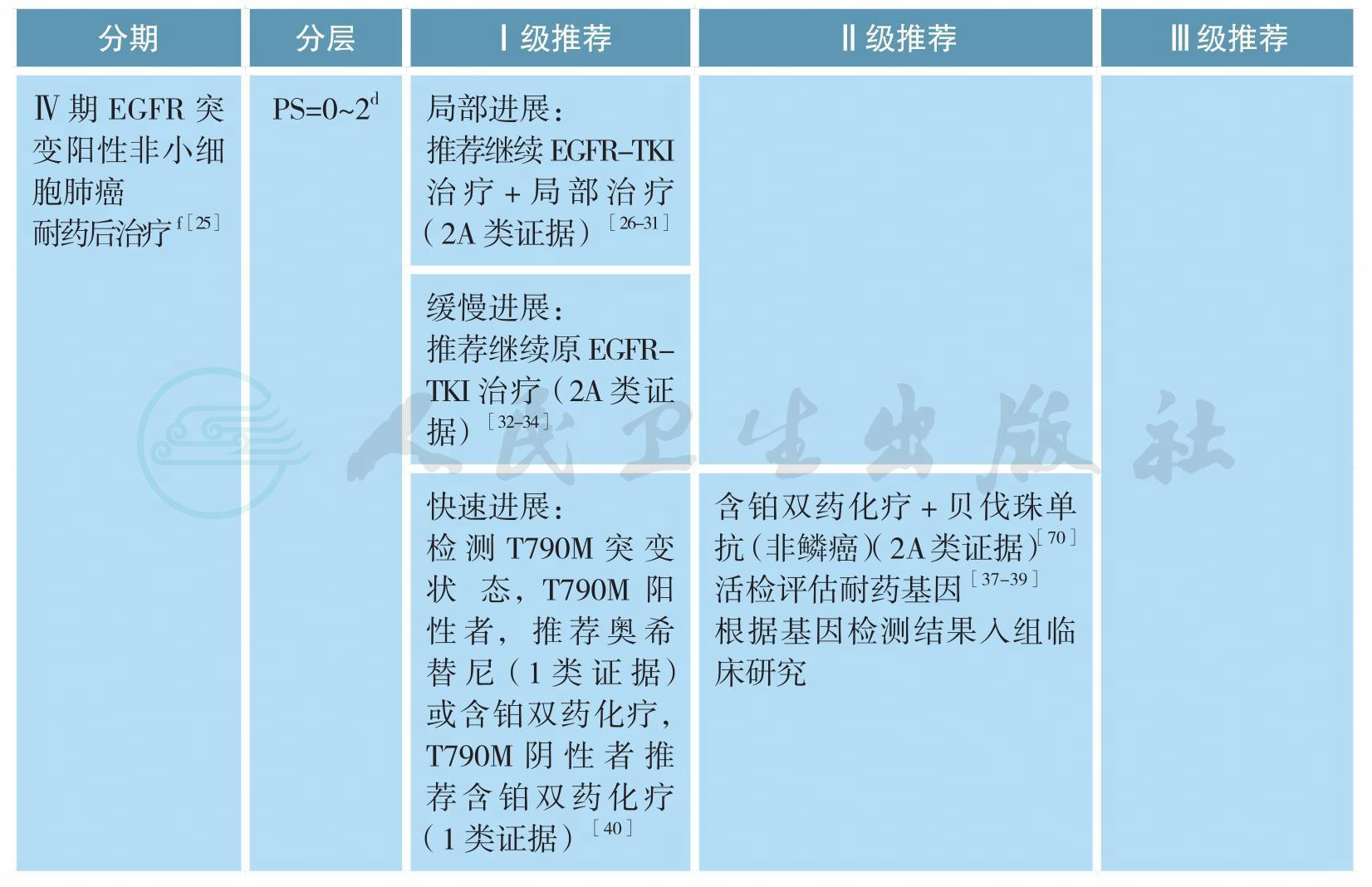
Ⅳ期驱动基因阳性非小细胞肺癌的治疗
EGFR突变患者的治疗

EGFR突变患者的治疗(续)
EGFR突变患者的治疗(续)

注:a.驱动基因阳性的鳞癌参照非鳞癌,本章节主要涉及多发转移患者,单发转移参考本指南其他相应章节;
b.确诊EGFR突变前由于各种原因接受了化疗的患者,在确诊EGFR突变后除推荐参考本指南选择EGFRTKI外,也可在疾病进展或不能耐受当前治疗后参考本指南一线治疗;
c.部分患者确诊晚期NSCLC后因为各种原因未能明确基因类型,一线接受化疗的患者进展后活检明确诊断为EGFR突变,治疗参考本指南一线治疗;
d.Ⅲ期临床研究均入组为PS≤2,EGFR-TKI在一线EGFR突变且PS=3分患者仅有Ⅱ期临床研究数据,具体请参考下述讨论部分;
e.基于经济原因或患者个人意愿,可参考本指南无驱动基因、Ⅳ期NSCLC治疗部分;
f.临床进展模式评估标准参考具体如下:
局部进展型:疾病控制≥3个月、颅外孤立进展或颅内进展、症状评分≤1;
缓慢进展型:疾病控制≥6个月、与以前相比,肿瘤负荷轻微增加、症状评分≤1;
快速进展型:疾病控制≥3个月、与以前相比,肿瘤负荷快速增加、症状评分2;
临床症状评分基于:5项与肺癌相关的临床表现(咳嗽、咳血、胸痛、发热和呼吸困难);1项转移灶相关的临床表现(如骨转移疼痛)组成;无症状为0分,稳定为1分,任一症状恶化或新发均为2分
ALK融合基因阳性或ROS1融合基因阳性非小细胞肺癌的治疗
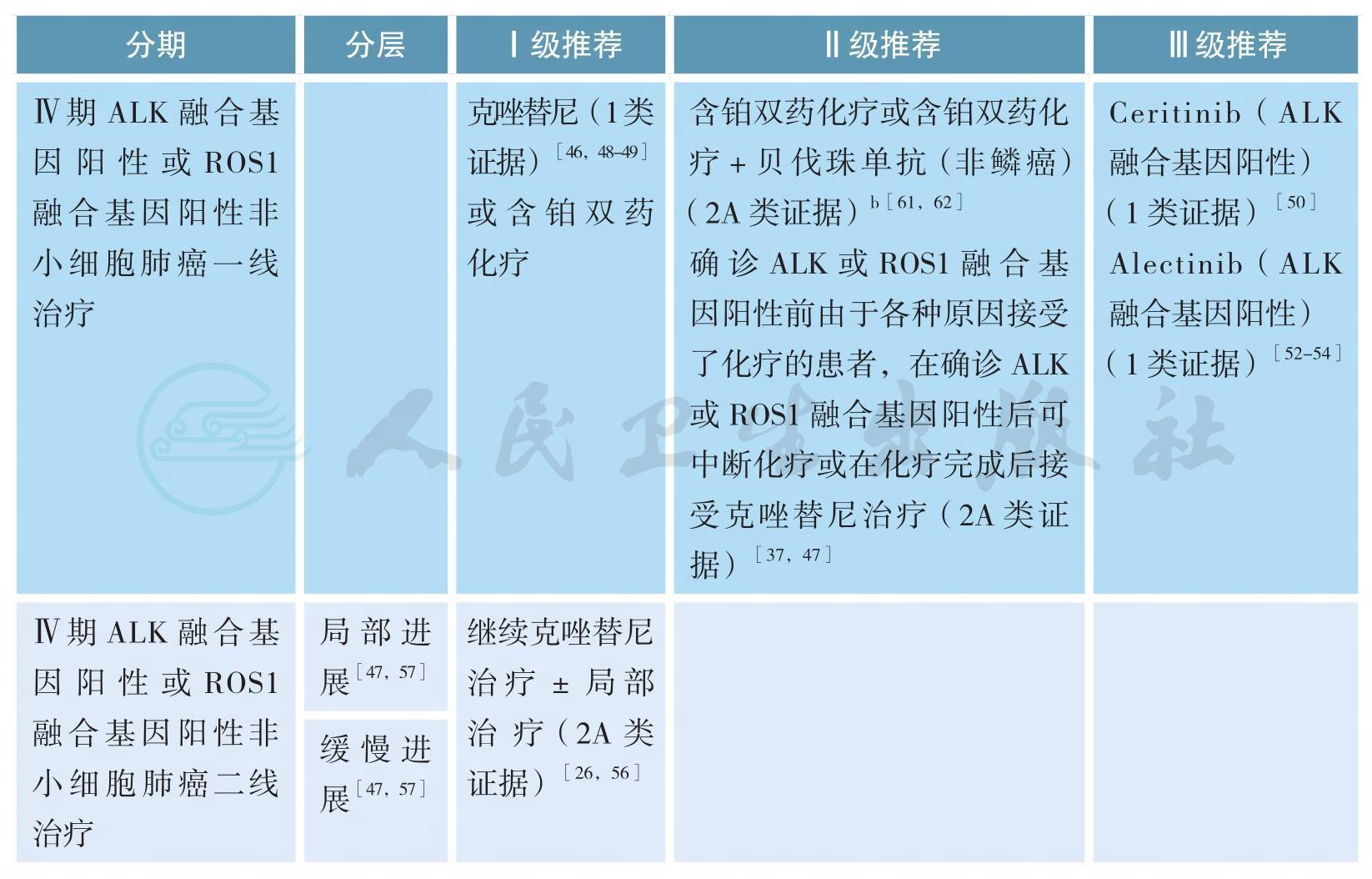
ALK融合基因阳性或ROS1融合基因阳性非小细胞肺癌的治疗(续)
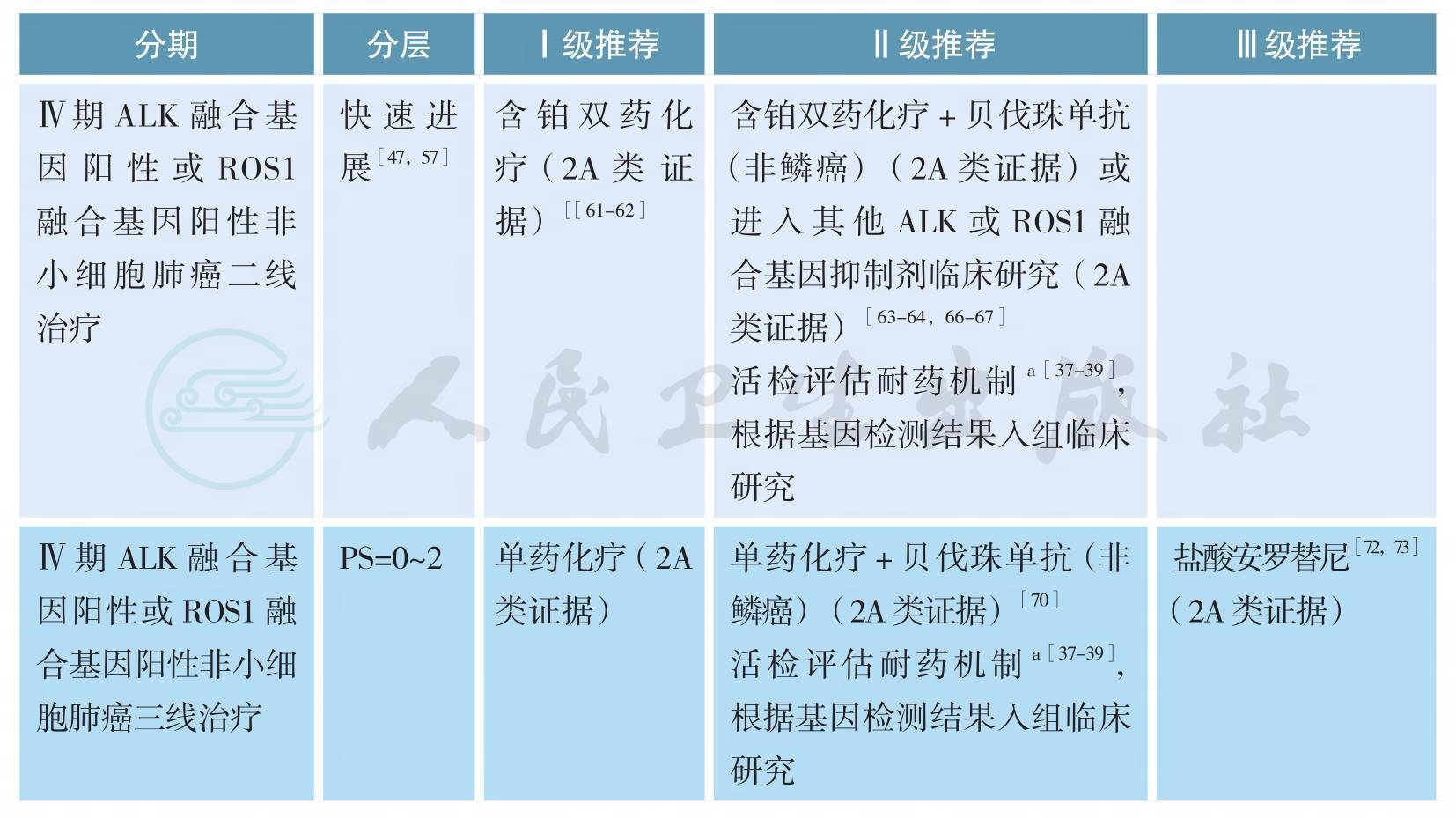
注:a.仅为基础或临床研究参考;
b.基于经济原因或患者个人意愿,非鳞癌推荐培美曲塞/铂类化疗方案
Ⅳ期无驱动基因、非鳞癌非小细胞肺癌的治疗
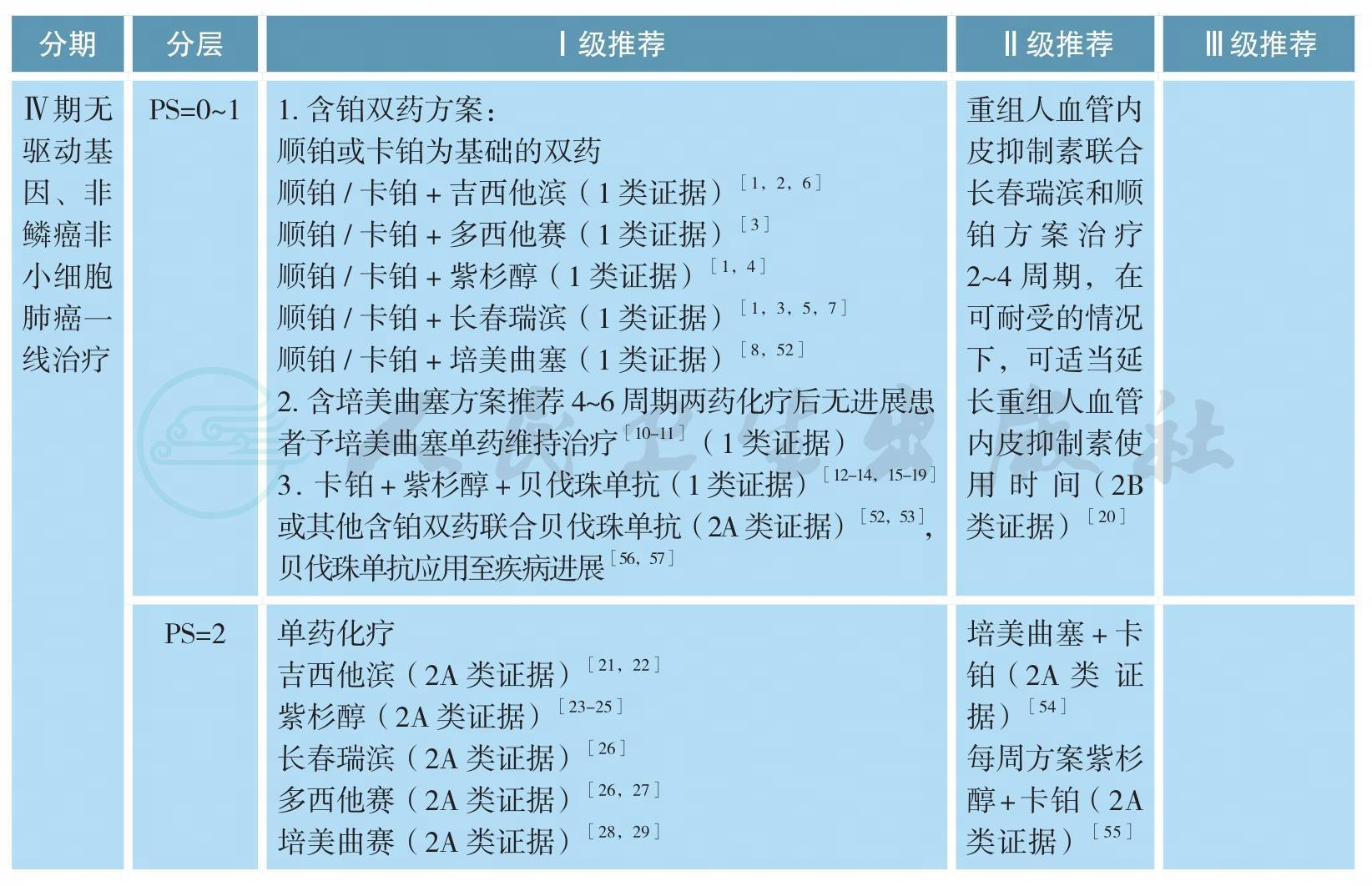
Ⅳ期无驱动基因、非鳞癌非小细胞肺癌的治疗(续)
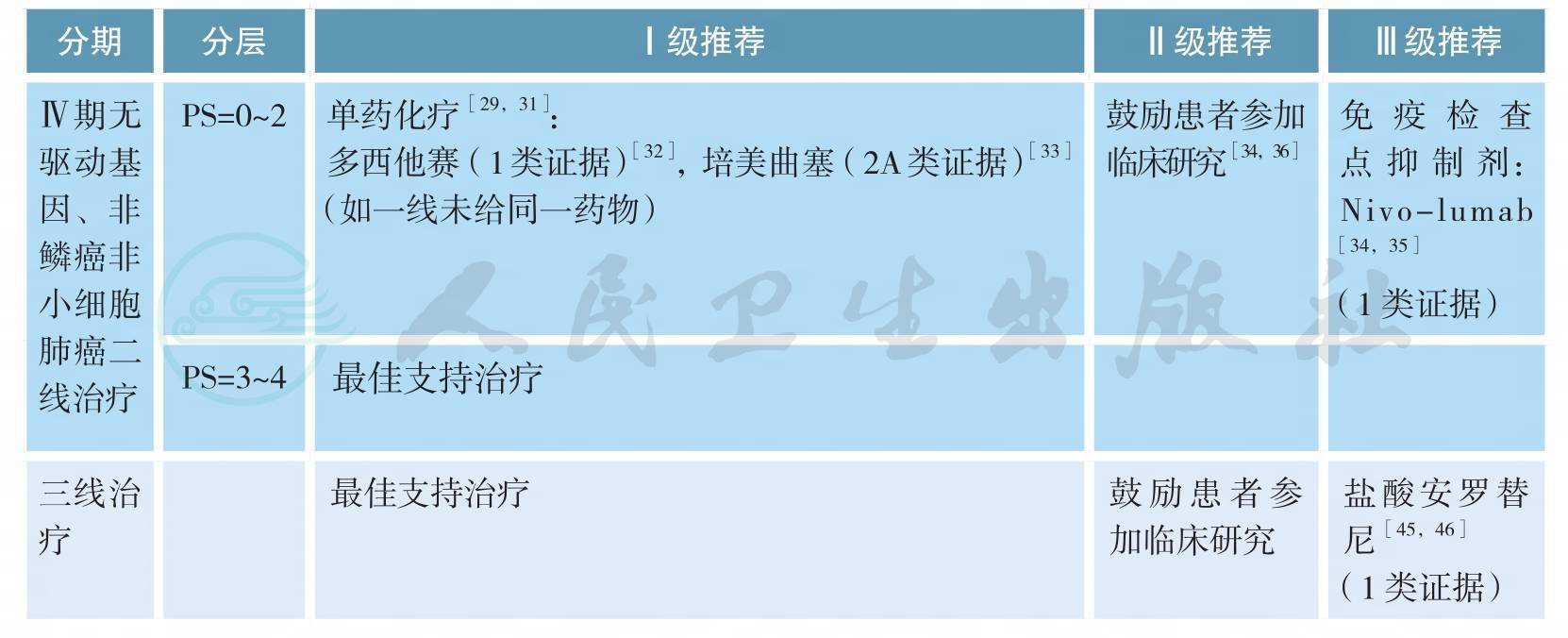
无驱动基因、Ⅳ期鳞癌的治疗
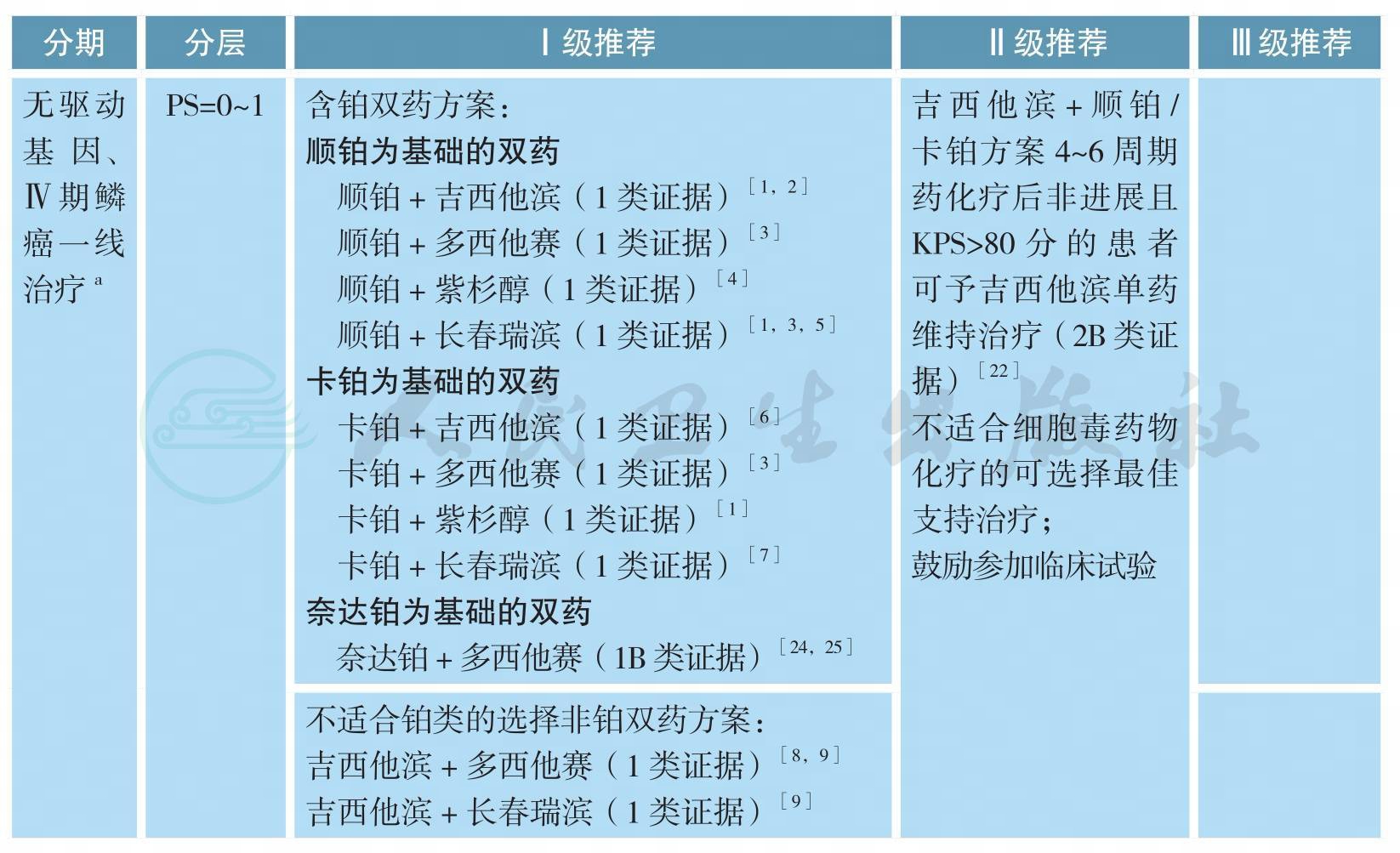
无驱动基因、Ⅳ期鳞癌的治疗(续)
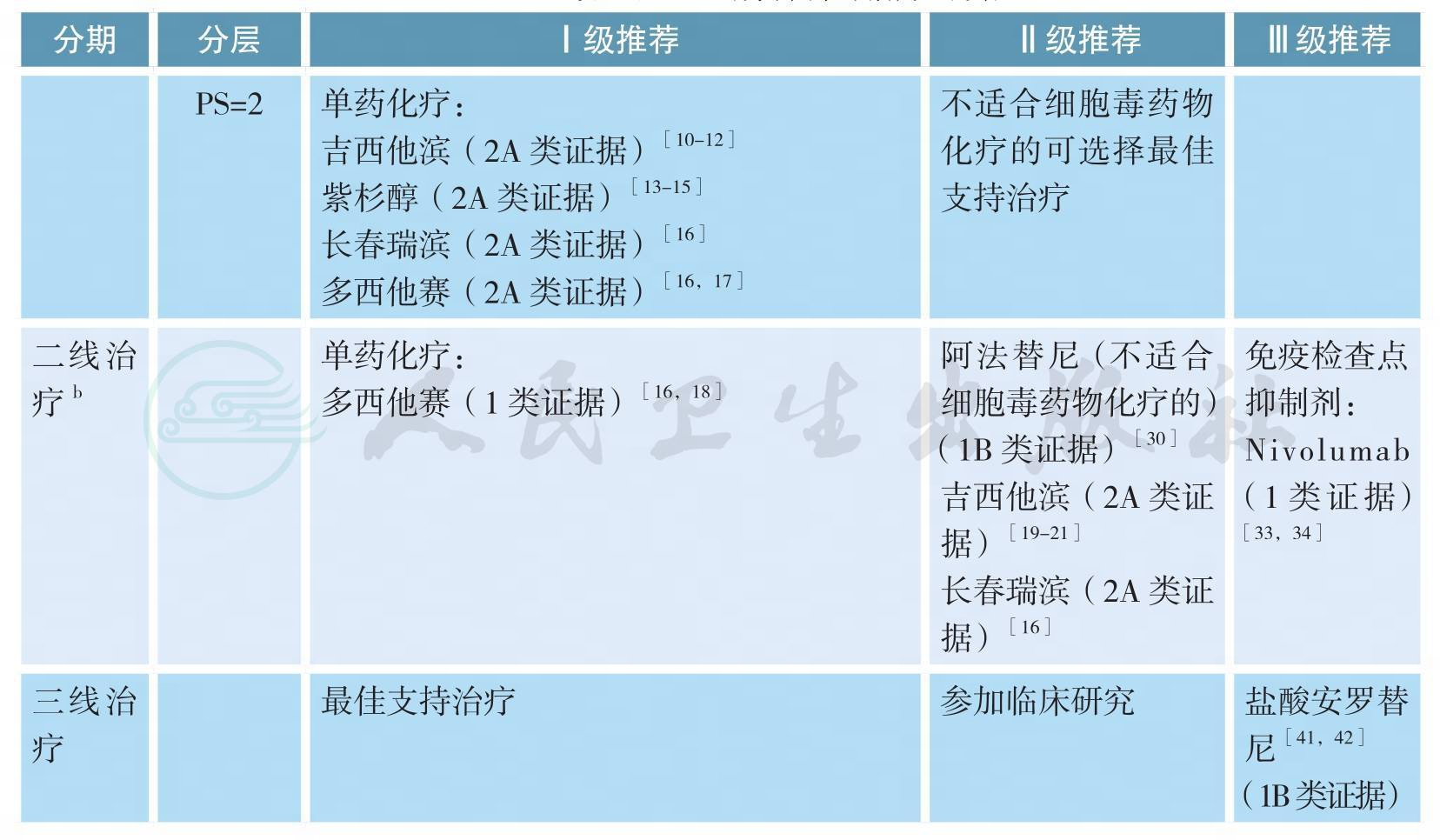
注:a.抗肿瘤治疗同时应给予最佳支持治疗;
b.如果疾病得到控制且毒性可耐受,可适当延长化疗周期数
Ⅳ期孤立性转移非小细胞肺癌的治疗
孤立脑或肾上腺转移NSCLC的治疗
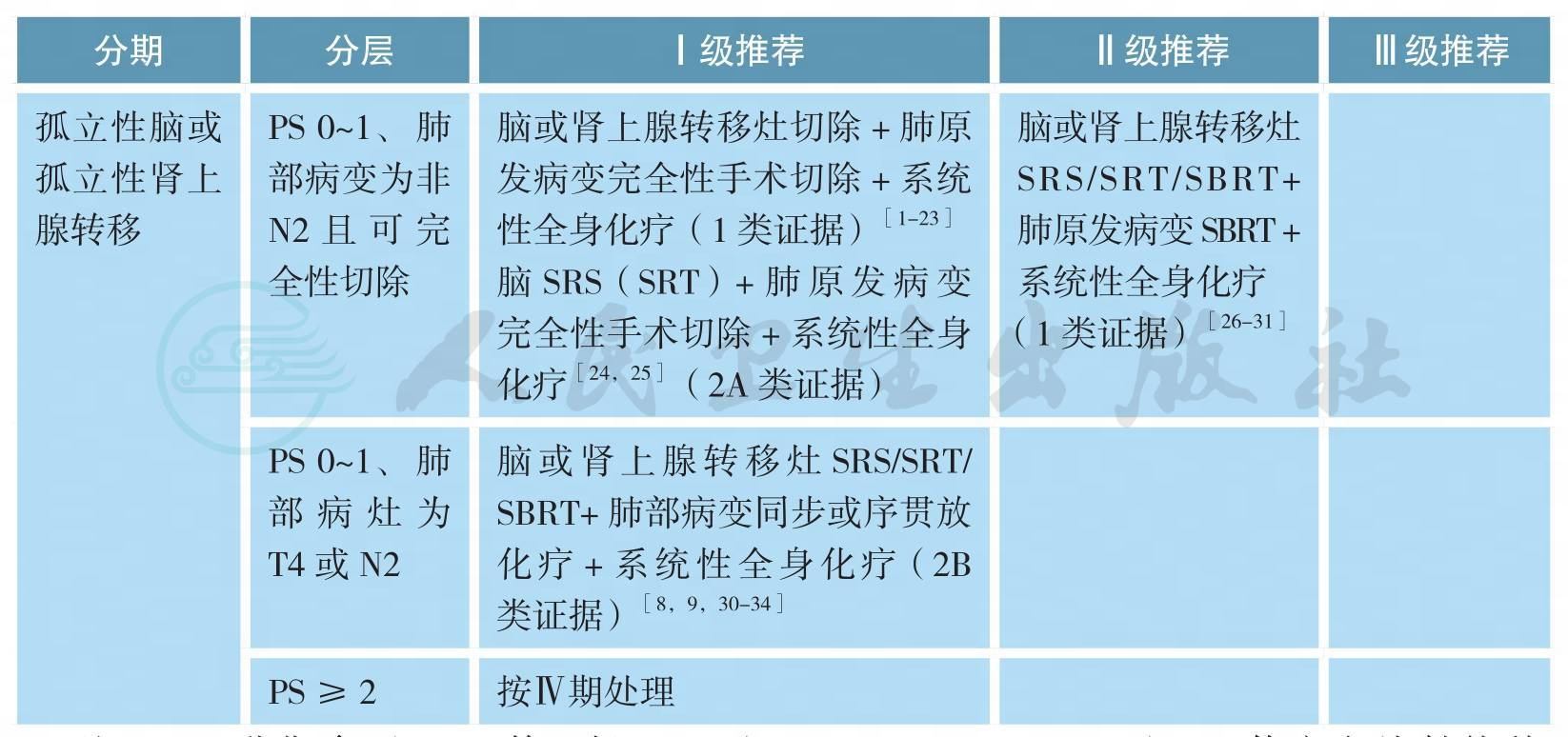
注:TNM分期参照UICC第7版;SRS(stereotactic radiosurgery):立体定向放射外科;WBRT(whole brain radiotherapy):全脑放射治疗;SRT(stereotactic radiation therapy):立体定向放疗;SBRT(stereotactic body radiation therapy):体部立体定向放疗
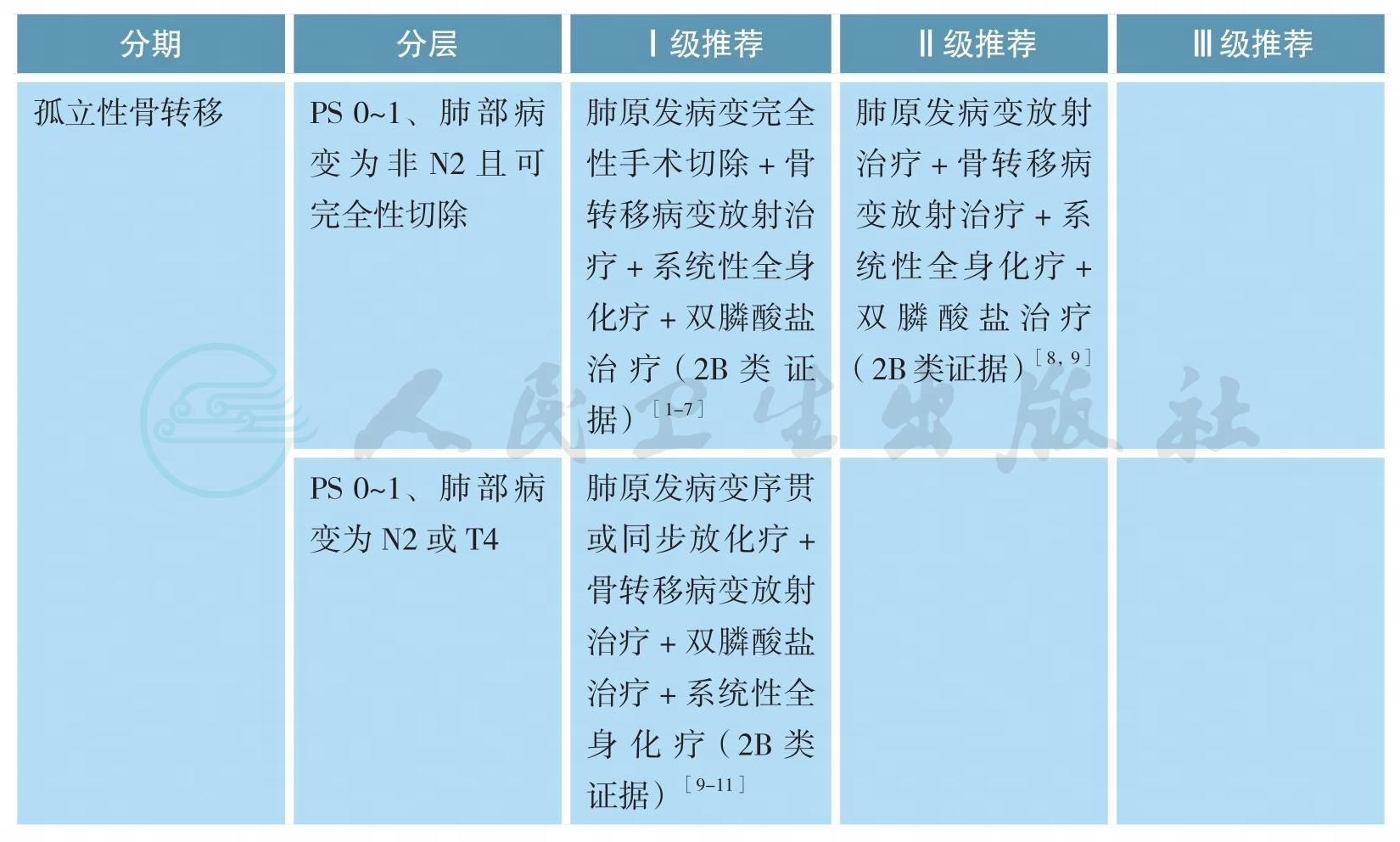
孤立性骨转移的处理
小细胞肺癌的治疗
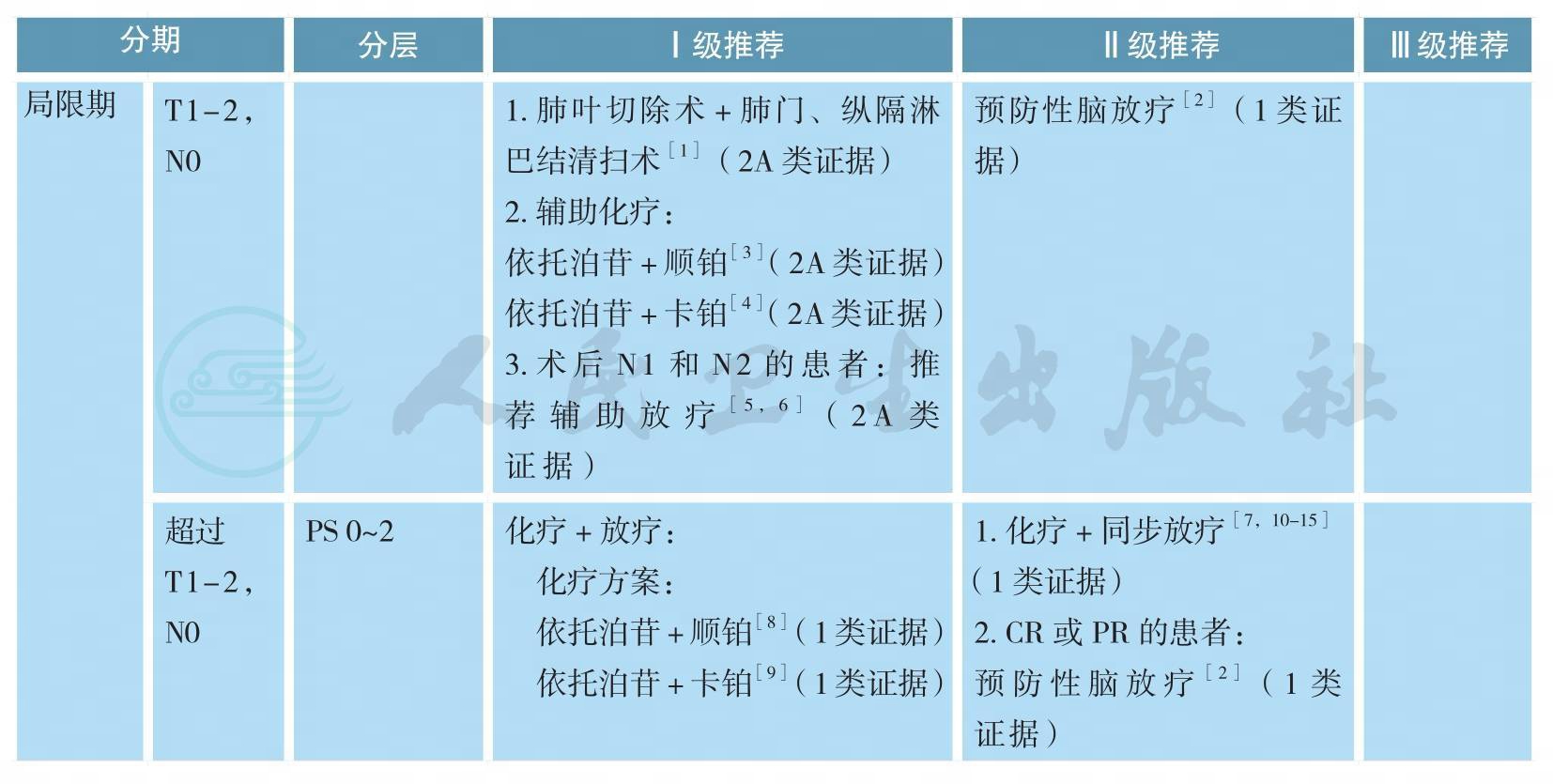
小细胞肺癌的治疗(续)
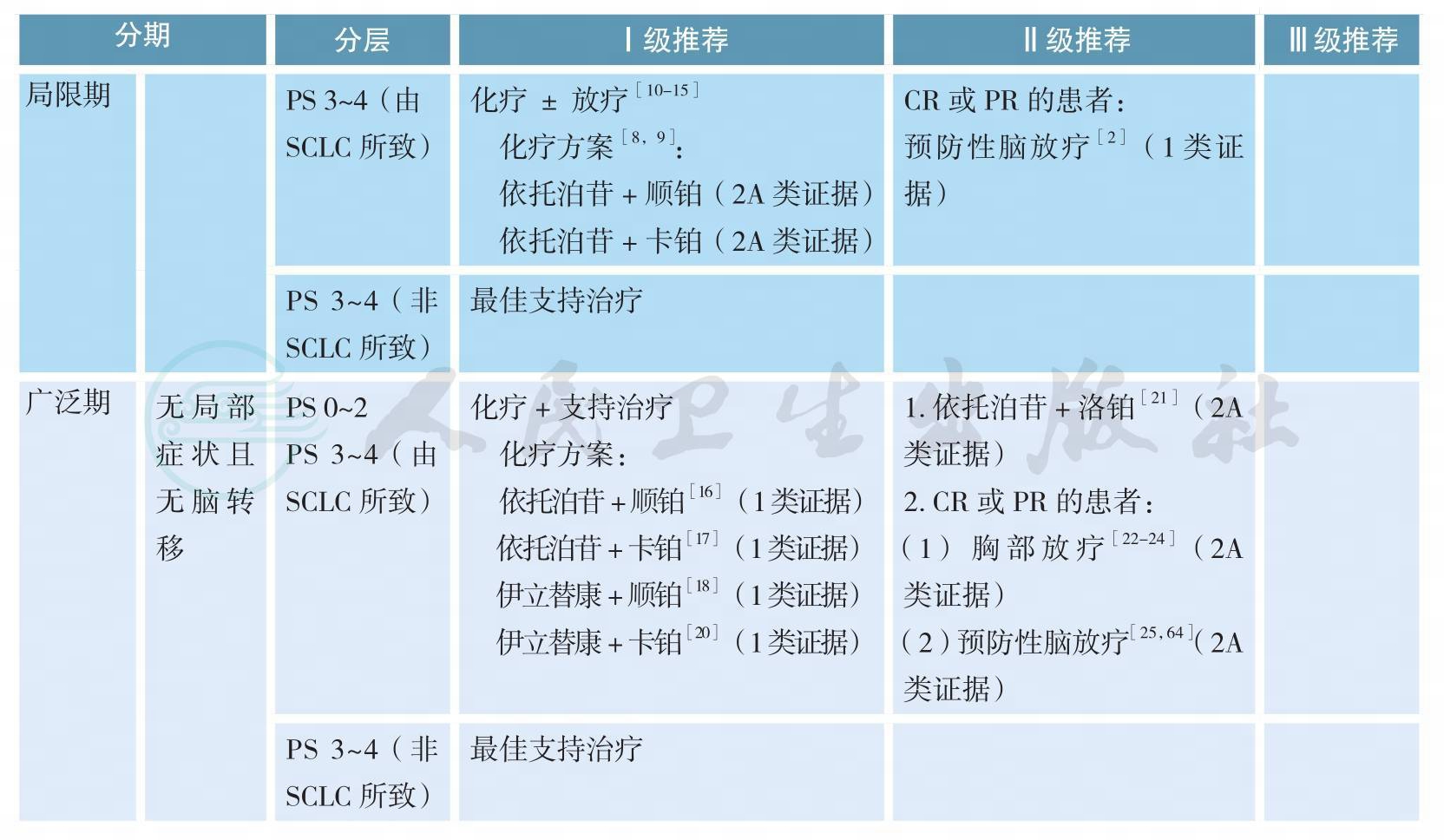
小细胞肺癌的治疗(续)
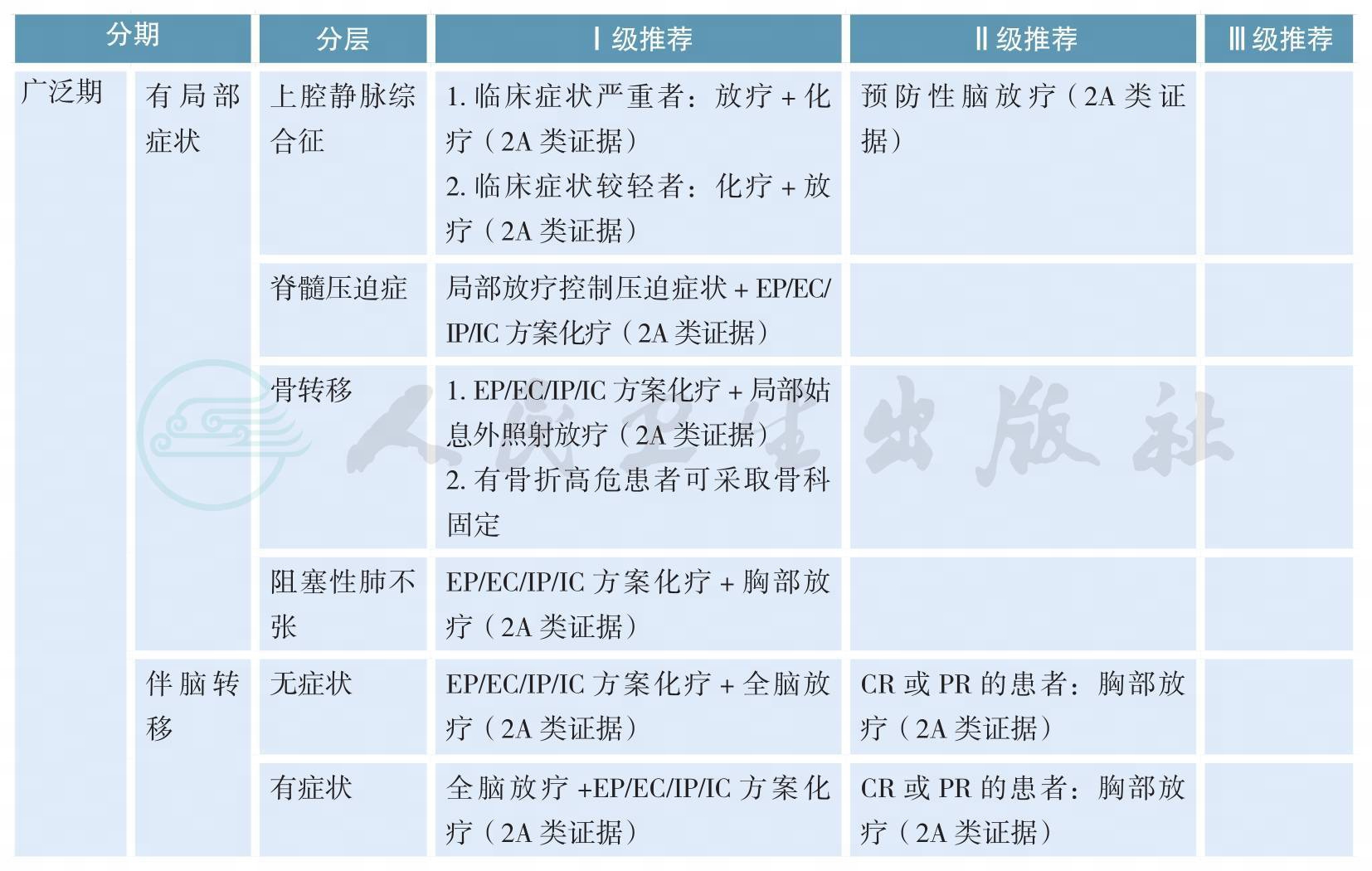
小细胞肺癌的治疗(续)

随访
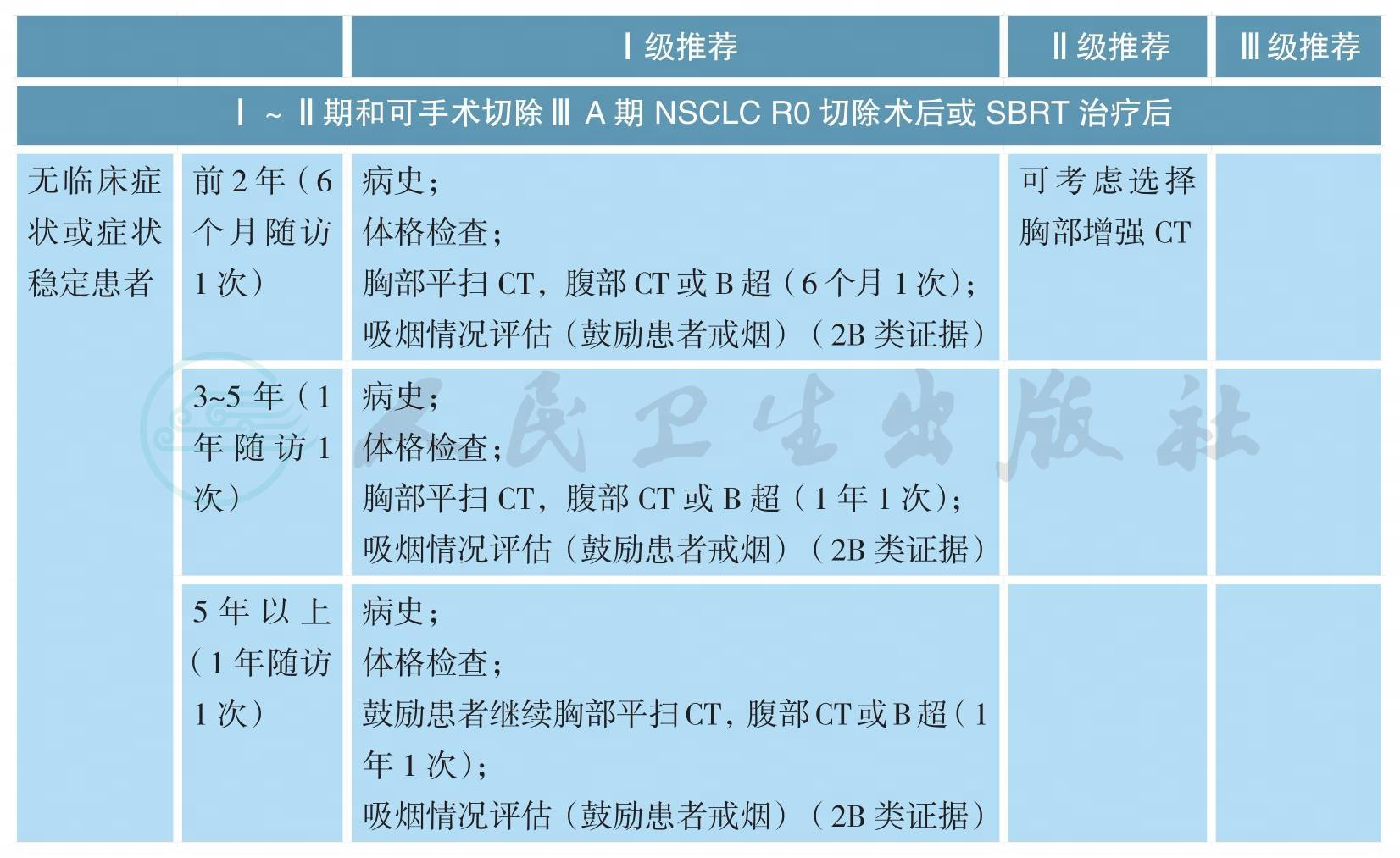
随访(续)
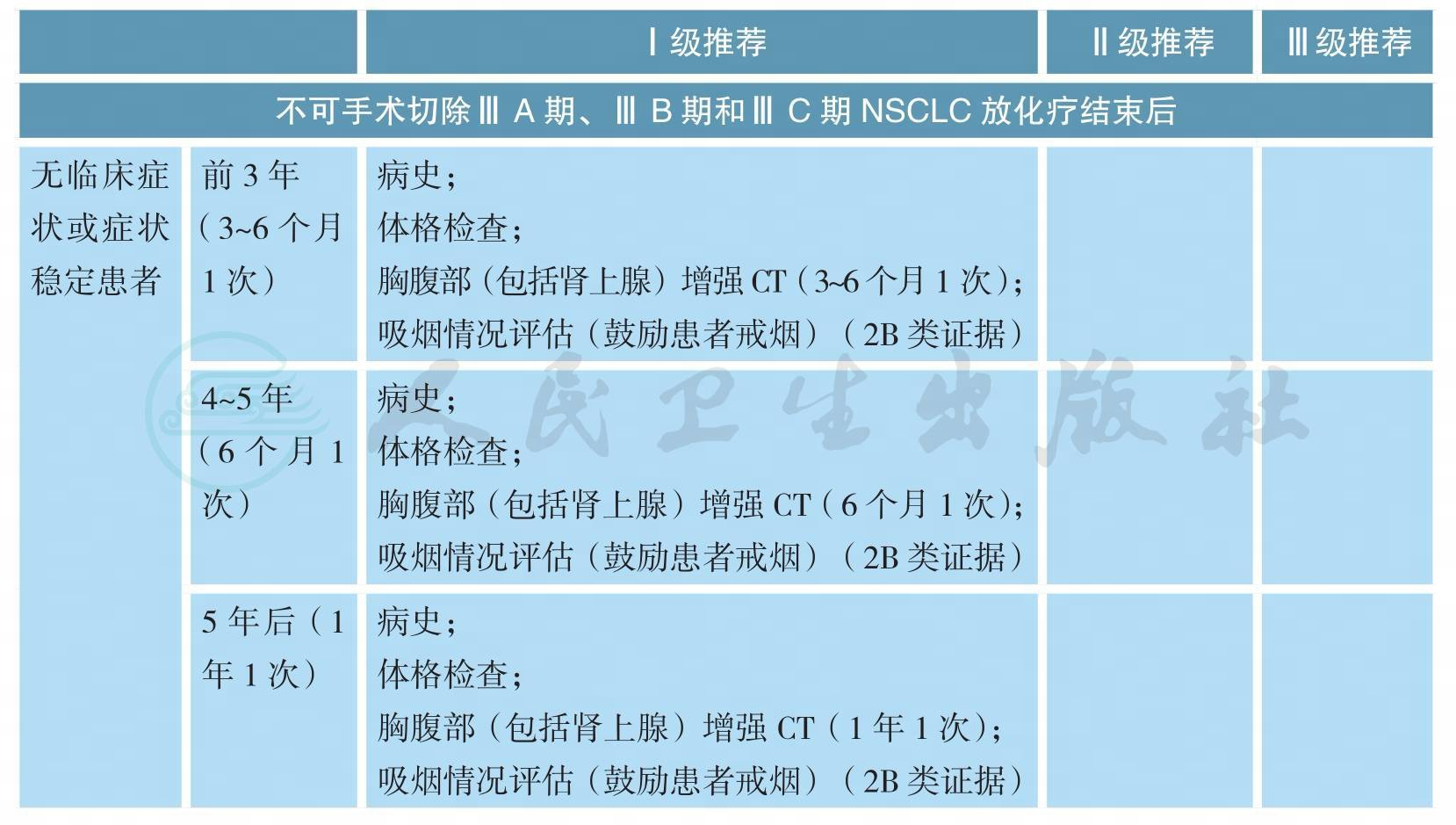
随访(续)

注:I~ⅢA期 NSCLC局部治疗后随访,常规不进行头颅CT或MRI、骨扫描或全身PET/CT检查,仅当患者出现相应部位症状时才进行;ⅢB~Ⅳ期 NSCLC不建议患者采用PET/CT检查作为常规复查手段
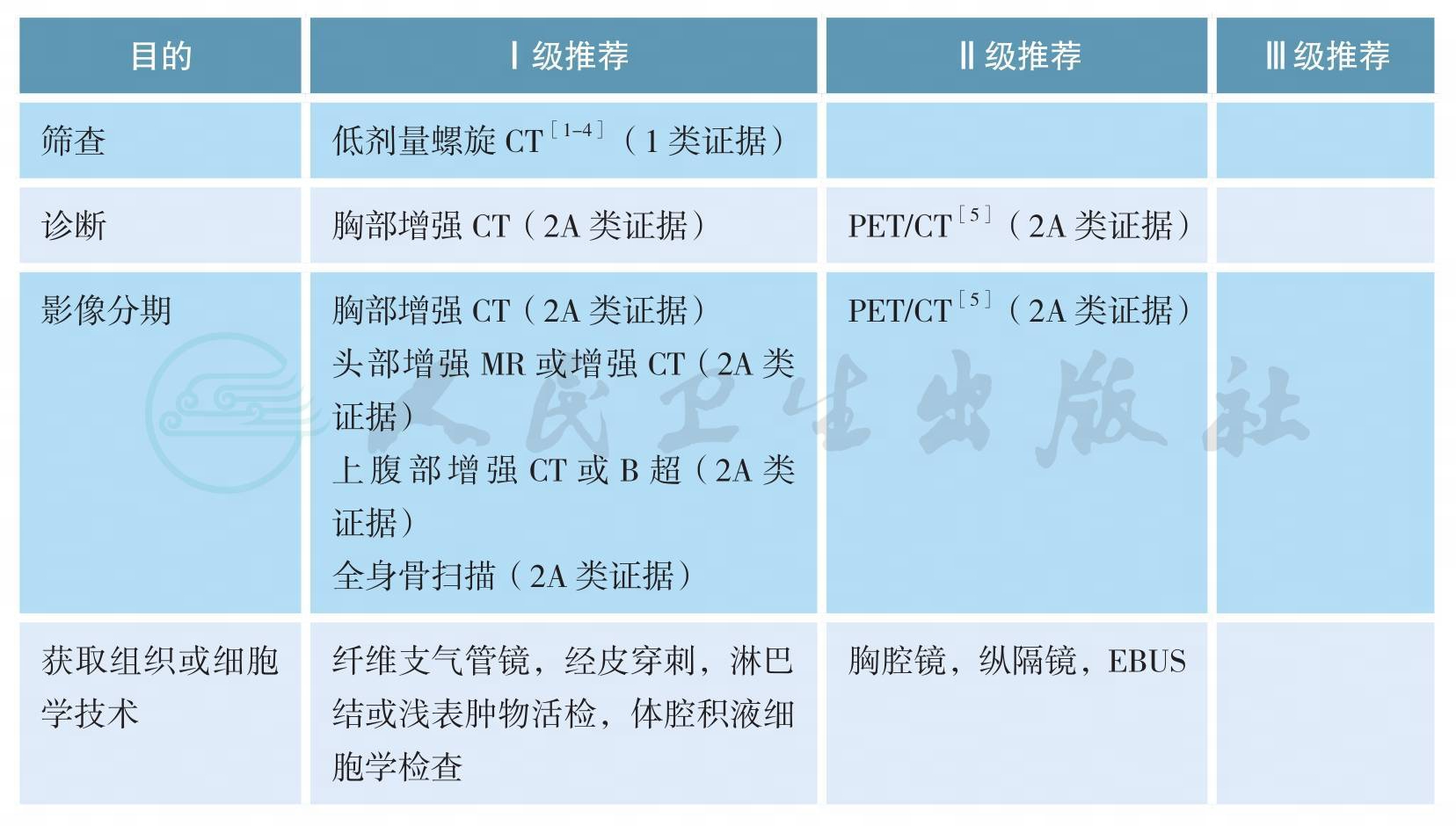
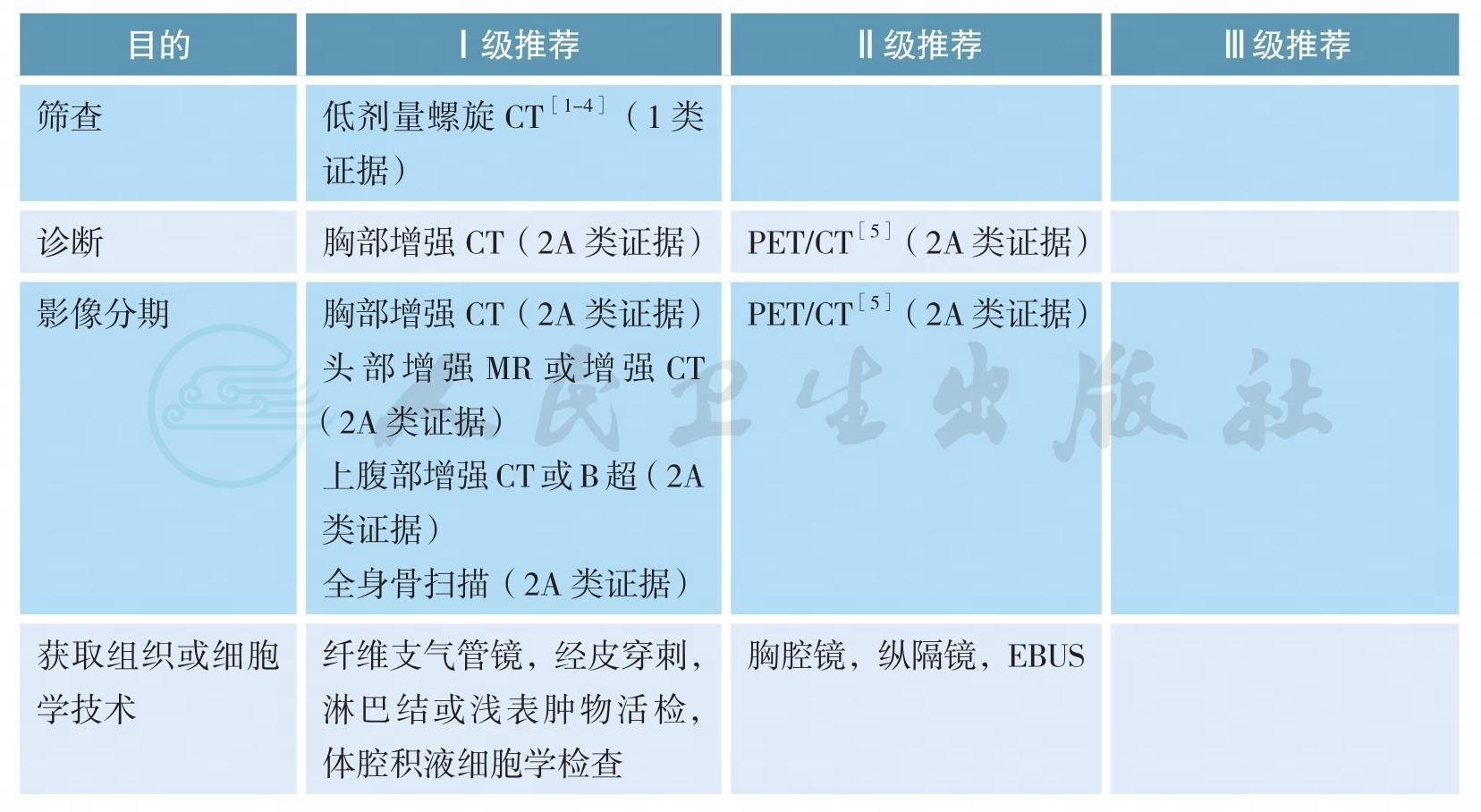
二、影像和分期诊断
影像和分期诊断
【注释】
肺癌是中国和世界范围内发病率和病死率最高的肿瘤,确诊时多数患者分期较晚是影响肺癌预后的重要原因[6,7],而早期肺癌可以通过多学科综合治疗实现较好的预后,甚至达到治愈的目的。因此,对高危人群进行肺癌筛查的研究一直在进行中。美国国家肺筛查试验(National Lung Screening Trial,NLST)纳入53454名重度吸烟患者进行随机对照研究,评估采用胸部低剂量螺旋CT筛查肺癌的获益和风险[1],结果显示,与胸片相比,经低剂量螺旋CT筛查的、具有高危因素的人群肺癌相关病死率降低了20%(95%CI6.8~26.7;P=0.004)[2]。此处高危人群指的是年龄在55~74岁,吸烟≥30包/年,仍在吸烟或者戒烟<15年(1类证据);年龄≥50岁,吸烟≥20包/年,另需附加一项危险因素(ⅡA类证据),危险因素包括:氡气暴露史,职业暴露史,恶性肿瘤病史,一级亲属肺癌家族史,慢性阻塞性肺气肿或肺纤维化病史[4]。推荐对高危人群进行低剂量螺旋CT筛查。
胸部增强CT、上腹部增强CT(或B超)、头部增强MR(或增强CT)以及全身骨扫描是肺癌诊断和分期的主要方法。一项Meta分析汇集了56个临床研究共8699例患者[6],结果提示,18F-FDG PET/CT对于淋巴结转移和胸腔外转移(脑转移除外)有更好的诊断效能。由于PET/CT价格昂贵,故本指南将PET/CT作为诊断和分期的可选策略。当纵隔淋巴结是否转移影响治疗决策,而其他分期手段难以确定时,推荐采用纵隔镜或超声支气管镜检查(EBUS)等有创分期手段明确纵隔淋巴结状态。
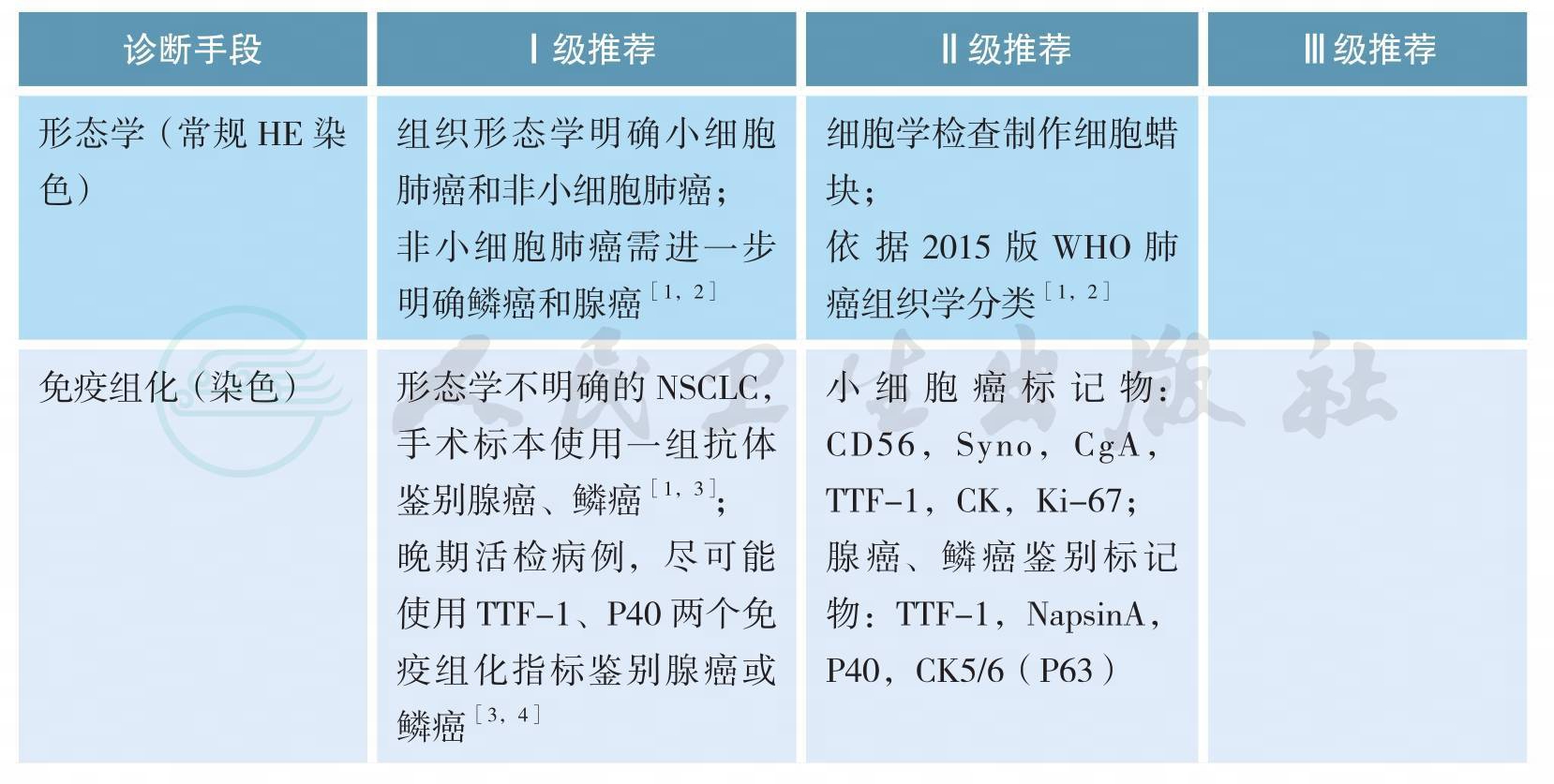
三、病理学诊断
病理学诊断
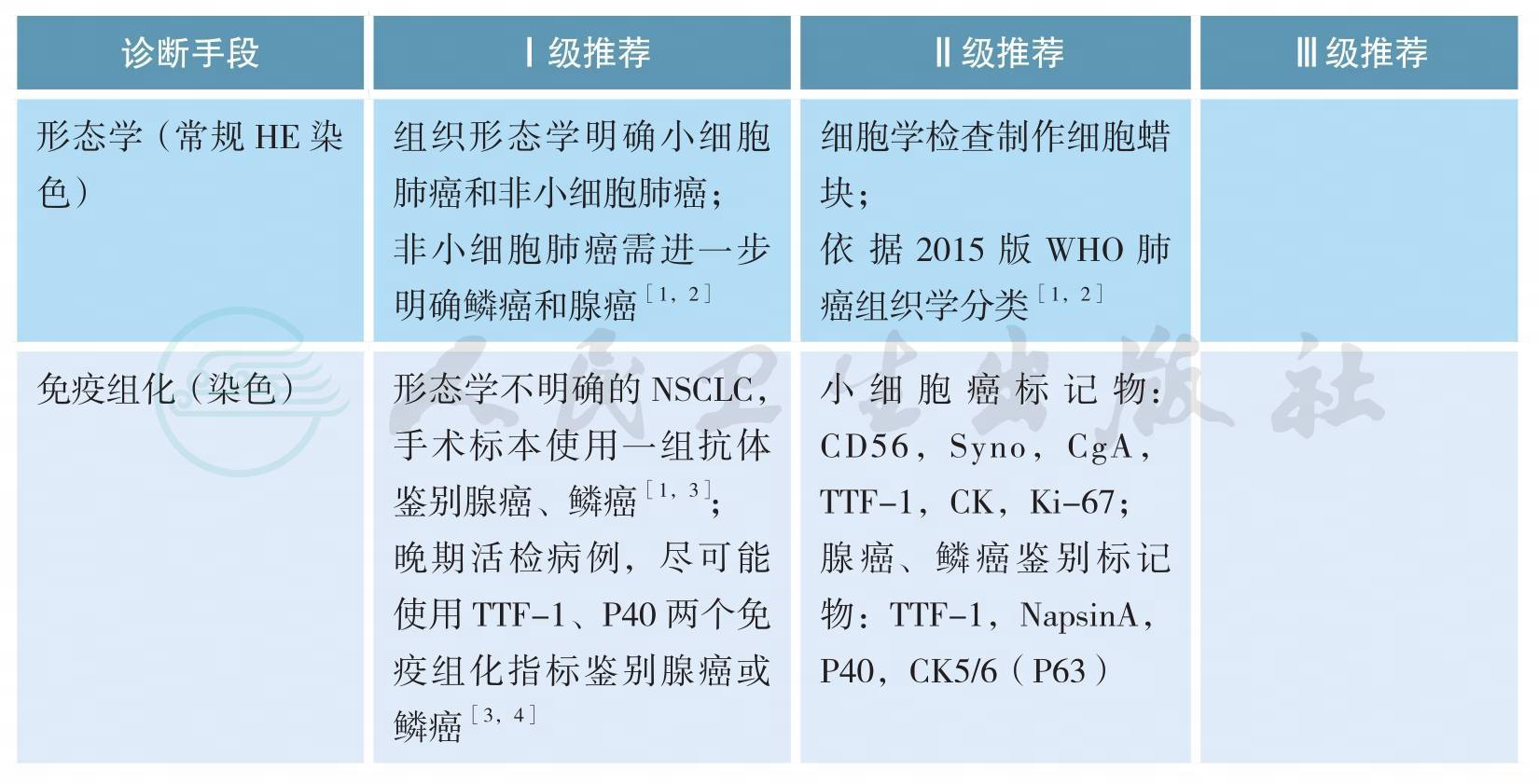
上述证据级别全部为2A类证据
【注释】
细胞学标本诊断原则
1.对找到肿瘤细胞或可疑肿瘤细胞标本均应尽可能制作与活检组织固定程序规范要求一致的FFPE细胞学蜡块。
2.根据细胞学标本形态特点及IHC染色结果可以对细胞学标本进行准确诊断、分型及细胞来源判断[5-7],与组织标本诊断原则类似,此类标本应尽量减少使用NSCLC-NOS(not otherwise specified)的诊断。细胞学标本分型及来源判断所采用的IHC染色指标及结果判读同组织学标本。
3.细胞学标本准确分型需结合免疫细胞化学染色,建议非小细胞肺癌细胞学标本病理分型不宜过于细化,仅作腺癌、鳞癌、神经内分泌癌或NSCLC- NOS等诊断,目前无需在此基础上进一步分型及进行分化判断。在细胞学标本不进行大细胞癌诊断[1]。
4.细胞学标本可以接受“可见异型细胞”病理诊断,并建议再次获取标本以明确诊断,但应尽量减少此类诊断。
5.各种细胞学制片及FFPE细胞学蜡块标本经病理质控后,均可进行相关驱动基因改变检测[8,9]。
组织标本诊断原则
1.手术标本及活检小标本诊断术语依据2015版WHO肺癌分类标准,见附录(病理诊断);手术切除标本诊断报告应满足临床分期及诊治需要。
2.临床医生应用“非鳞癌”界定数种组织学类型及治疗相似的一组患者,在病理诊断报告中应将NSCLC分型为腺癌、鳞癌、NSCLC-NOS及其他类型,不能应用“非鳞癌”这一术语。
3.如果同时有细胞学标本及活检标本时,应结合两者观察,综合做出更恰当诊断。
4.原位腺癌(AIS)及微小浸润癌(MIA)的诊断不能在小标本及细胞学标本完成,术中冰冻诊断也有可能不准确。如果在小标本中没有看到浸润,应归为肿瘤的贴壁生长方式,可诊断为腺癌,并备注不除外AIS、MIA或贴壁生长方式的浸润性腺癌[1]。<3cm临床表现为毛玻璃影成分的肺结节手术切除标本应全部取材,方可诊断AIS或MIA。
5.手术标本腺癌需确定具体病理亚型及比例(以5%含量递增比例)。按照各亚型所占比例从高至低依次列出。微乳头型腺癌及实体型腺癌未达5%亦应列出。
6.腺鳞癌诊断标准为具有鳞癌及腺癌形态学表现或免疫组化标记显示有两种肿瘤类型成分,每种类型至少占10%以上。小标本及细胞学标本不能做出此诊断。
7.神经内分泌免疫组化检测只应用于肿瘤细胞形态学表现出神经内分泌特点的病例。
8.同一患者治疗后不同时间小标本活检病理诊断尽量避免使用组织类型之间转化的诊断[10],如小细胞癌,治疗后转化为非小细胞癌。此种情况不能除外小活检标本取材受限,未能全面反映原肿瘤组织学类型,有可能原肿瘤是复合性小细胞癌,化疗后其中非小细胞癌成分残留所致。
9.神经内分泌肿瘤标记物包括CD56、Syn、CgA,在具有神经内分泌形态学特征基础上至少有一种神经内分泌标记物明确阳性,神经内分泌标记阳性的细胞数应大于10%肿瘤细胞量才可诊断神经内分泌肿瘤。在少量SCLC中可以不表达神经内分泌标记物,结合形态及TTF-1弥漫阳性与CK核旁点状阳性颗粒特点也有助于SCLC的诊断[10]。
10.怀疑累及肺膜时,应进行弹力纤维特殊染色辅助判断[11,12];特染AB/PAS染色、黏液卡红染色用于判断黏液分泌;腺癌鉴别指标:TTF-1,Napsin-A;鳞癌:P40,P63,CK5/6,注意P63也可表达于部分肺腺癌中,相对来讲,P40、CK5/6对鳞状细胞癌更特异[1-4]。
11.对于晚期NSCLC患者小标本,尽可能少地使用免疫组化指标(TTF-1,P40)以节省标本用于后续分子检测[1,4,13]。
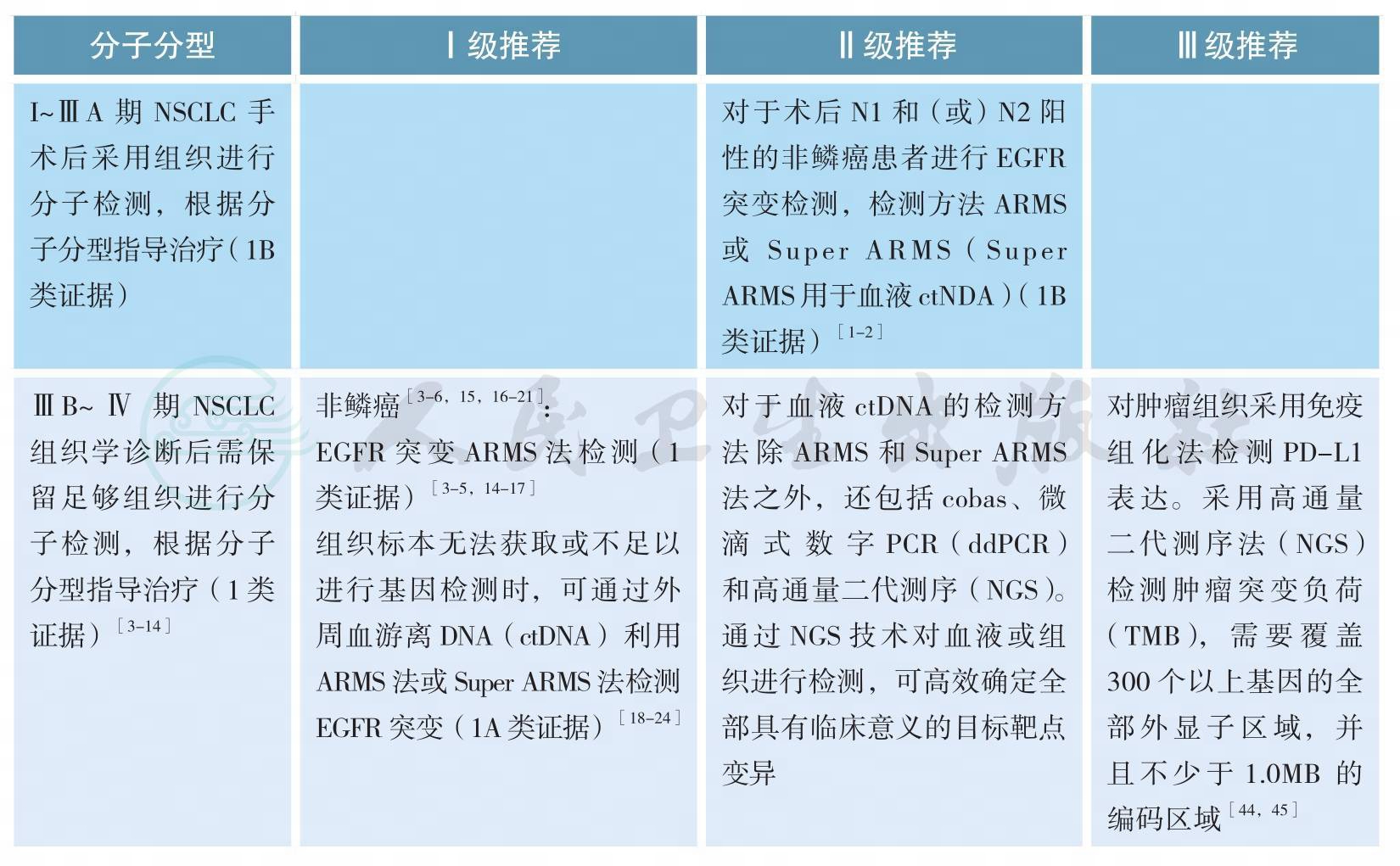
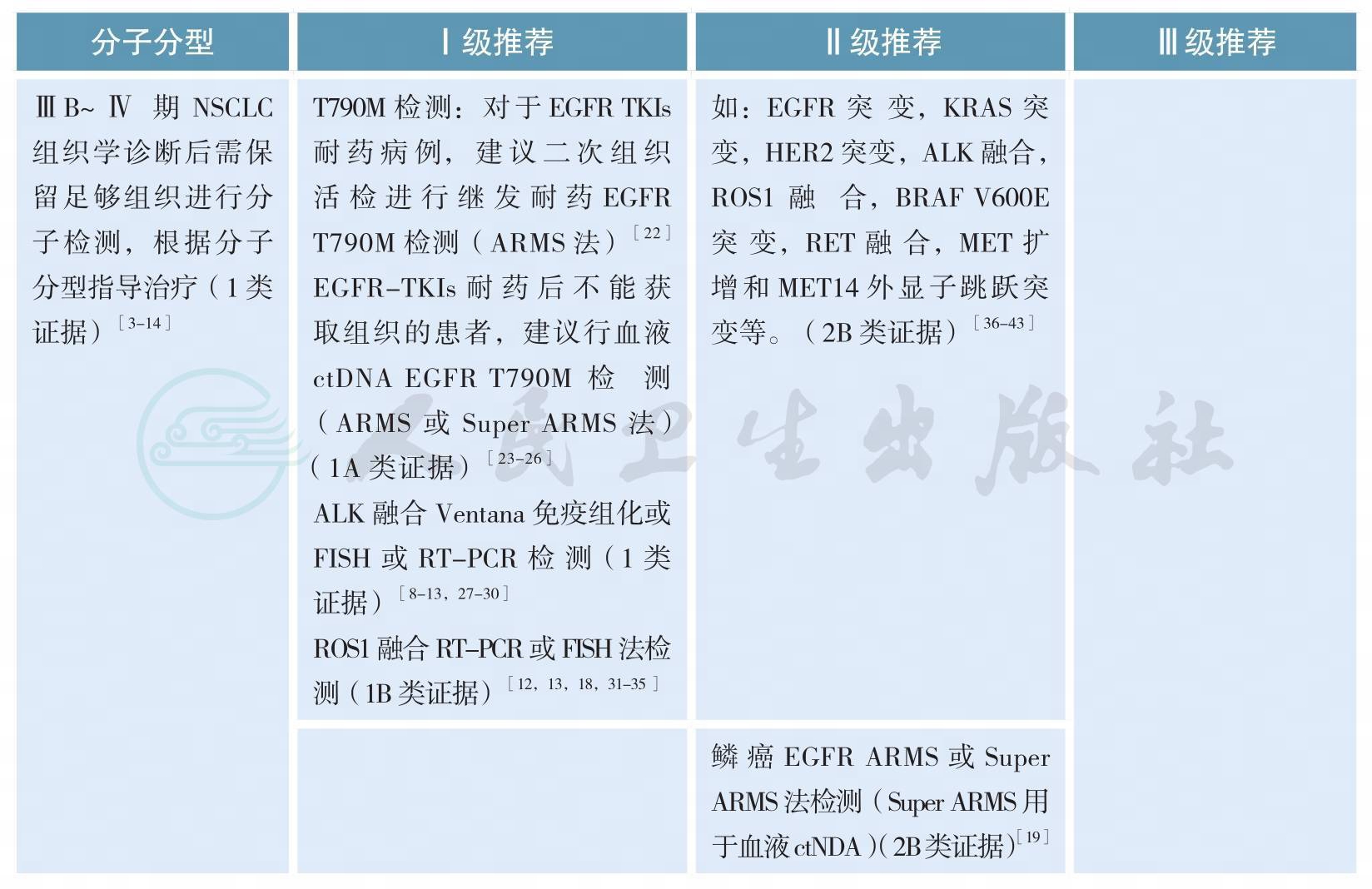
四、分子分型
分子分型
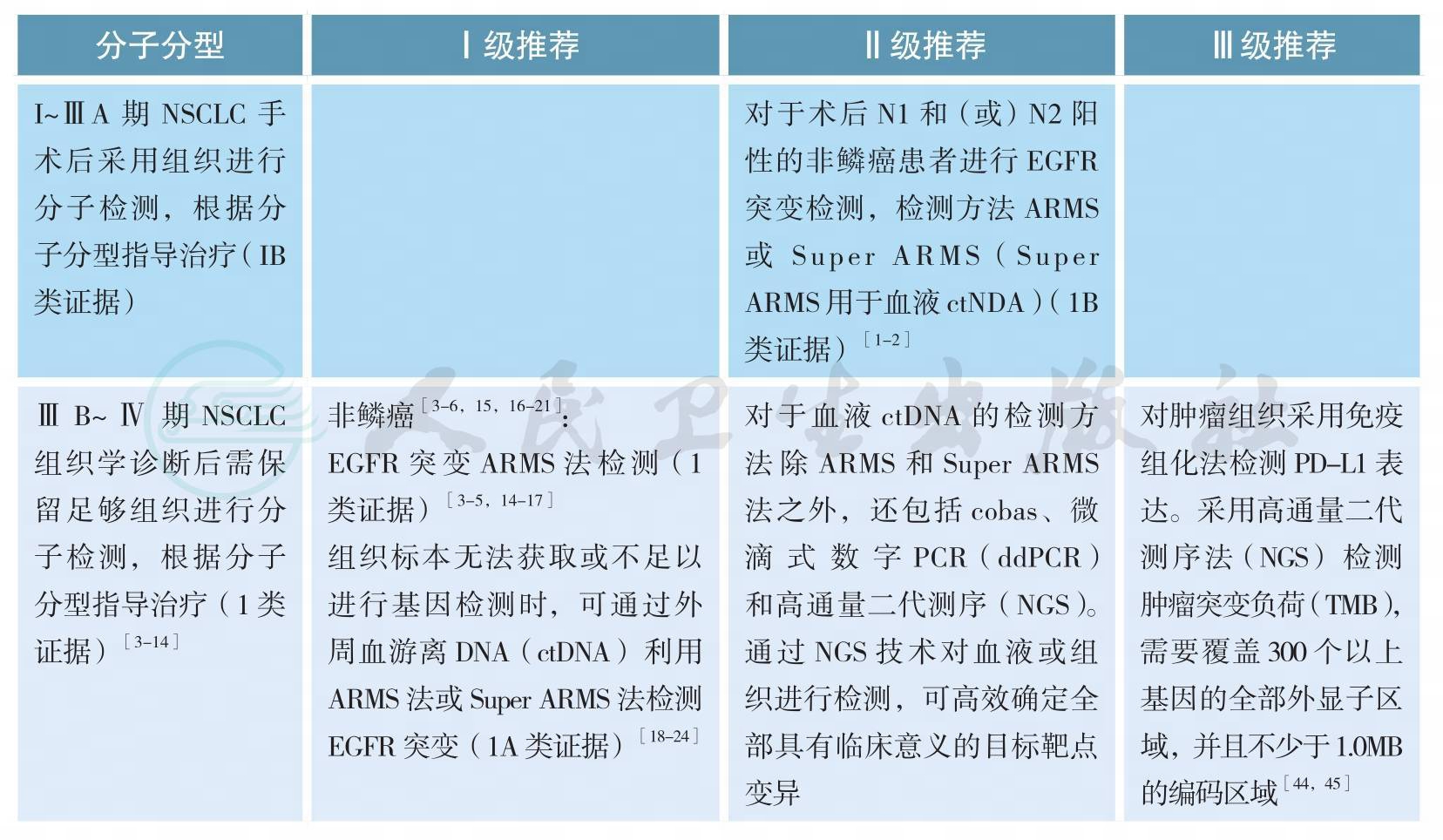
分子分型(续)
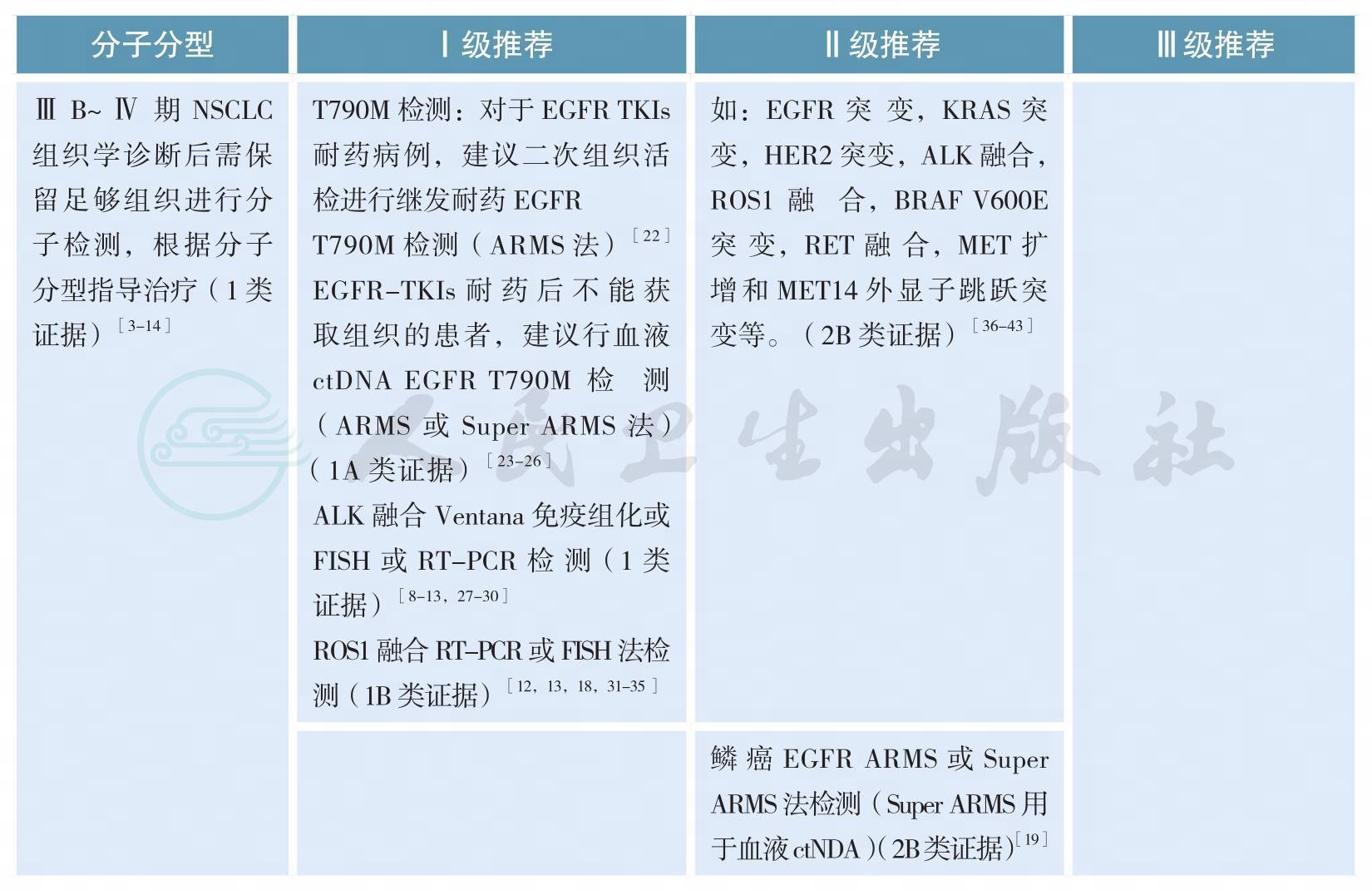
【注释】
随着肺癌系列致癌驱动基因的相继确定,我国及国际上多项研究表明靶向治疗药物大大改善和延长携带相应驱动基因的非小细胞肺癌(non-small cell lung cancer,NSCLC)患者的预后和生存[3-7]。肺癌的分型也由过去单纯的病理组织学分类,进一步细分为基于驱动基因的分子亚型[10-12]。晚期表皮生长因子受体(epidermal growth factor receptor,EGFR)敏感突变和间变性淋巴瘤激酶(anaplasticlymphoma kinase,ALK)阳性NSCLC精准靶向治疗的疗效与分子分型关系已经在临床实践中得到充分证实[3-9]。今年Lancet Oncology发表的重要研究CTONG1104以及世界肺癌大会(WCLC)报道的EVAN研究结果支持早期术后具有N1N2的非鳞NSCLC患者进行EGFR突变检测,因术后辅助EGFR酪氨酸激酶抑制剂(EGFR-TKIs)治疗为这部分患者带来了获益[1-2]。
所有含腺癌成分的NSCLC,无论其临床特征(如吸烟史,性别,种族,或其他等),应常规进行EGFR突变/ALK融合基因检测,EGFR突变检测应涵盖EGFR 18、19、20、21外显子,ALK和ROS1的检测应与EGFR突变检测平行进行[11]。尤其在标本量有限的情况下,可采用经过验证的检测方法同时检测多个驱动基因的技术,如多基因同时检测的PCR技术或二代测序技术(next generation sequencing,NGS)等。
EGFR突变/ALK融合/ROS1融合的检测应在患者诊断为晚期NSCLC时立即进行,早期肺癌患者演变为Ⅳ期时也应进行EGFR突变/ALK融合/ROS1融合的检测[13]。
原发肿瘤和转移灶都适于进行EGFR突变/ALK融合/ROS1融合分子检测[13]。
为了避免样本浪费和节约检测时间,对于晚期NSCLC活检样本,应根据所选用的技术特点,一次性切出需要诊断组织学类型和进行EGFR突变/ALK融合/ROS1融合检测的样本量,避免重复切片浪费样本;如果样本不足进行分子检测,建议进行再次取材,确保分子检测有足够样本。
难以获取肿瘤组织样本时,多项回顾性大样本研究显示外周血游离肿瘤DNA(cell-free tumor DNA,ctDNA)EGFR基因突变检测相较肿瘤组织检测,具有高度特异性(97.2%~100%)及对EGFR-TKIs疗效预测的准确性,但敏感度各家报道不一(50.0%~81.8%)[18-21]。欧洲药品管理局2014年9月已批准当难以获取肿瘤组织样本时,可采用外周血ctDNA作为补充标本评估EGFR基因突变状态,以明确最可能从吉非替尼治疗中受益的NSCLC患者。CFDA在2015年2月亦已批准对吉非替尼说明书进行更新,补充了如果肿瘤标本不可评估,则可使用从血液(血浆)标本中获得的ctDNA进行检测,但特别强调ctDNA EGFR突变的检测方法必须是已经论证的稳定、可靠且灵敏的方法,以避免出现假阴性和假阳性的结果。2018年初厦门艾德的Super-ARMS试剂盒已经获得中国食品药品管理局(CFDA)的批准,可用于ctDNA的基因检测;其他ctDNA的基因检测方法还包括cobas、微滴式数字PCR(droplet digital PCR,ddPCR)和NGS。因此,当肿瘤组织难以获取时,血液是EGFR基因突变检测合适的替代生物标本,也是对可疑组织检测结果的补充。T790M突变是一代EGFR-TKI主要耐药机制之一,占比超过50%,三代EGFR-TKI奥希替尼作用于该靶点,AURA3[23]已证实可有效治疗一代/二代EGFR-TKI治疗进展伴T790M突变患者,奥希替尼在中国已获CFDA批准用于T790M阳性的一代/二代EGFR-TKI耐药患者。研究报道血浆ctDNA可用来检测T790M突变[24],可作为二次活检组织标本不可获取的替代标本,同时也是对可以组织检测结果的补充。2017年在WCLC会议上报道的前瞻性BENEFIT研究,AURA3研究以及FLAURA研究的ctDNA分析结果再次证明了外周血基础上EGFR敏感突变和T790M耐药突变检测的可行性[23,25,-26]。采用脑脊液、胸腔积液上清等标本进行基因检测目前尚在探索中。
目前对于ALK/ROS1融合基因的血液检测,技术尚不成熟,因此对于ALK/ROS1融合基因检测,仍该尽最大可能获取组织或细胞学样本进行检测。
亚裔人群和我国的肺腺癌患者EGFR基因敏感突变阳性率为40%~50%[14-16]。EGFR突变主要包括4种类型:外显子19缺失突变、外显子21点突变、外显子18点突变和外显子20插入突变[17]。最常见的EGFR突变为外显子19缺失突变(19DEL)和外显子21点突变(21L858R),均为EGFRTKI的敏感性突变,18外显子G719X、20外显子S768I和21外显子L861Q突变亦均为敏感性突变,20外显子的T790M突变与第一、二代EGFR-TKI获得性耐药有关,还有许多类型的突变临床意义尚不明确[22]。
ALK阳性NSCLC的发生率为3%~7%,东西方人群发生率没有显著差异[27,28]。中国人群腺癌ALK阳性率为5.1%[28]。而我国EGFR和KRAS均为野生型的腺癌患者中ALK融合基因的阳性率高达30%~42%[27,28]。有研究表明,年龄是ALK阳性NSCLC一项显著的独立预测因子,基于我国人群的研究发现,在年龄小于51岁的年轻患者中,ALK重排的发生率高达18.5%;也有研究发现,在年龄小于40岁的年轻患者中,ALK重排的发生率近20%[27,28]。
从检测方法学角度考虑,ALK阳性NSCLC不仅是基因序列层面的改变即序列重排,ALK融合蛋白也是该类疾病中的重要变异。检测技术包括ALK基因FISH检测、或ALK融合变异RT-PCR检测、或ALK融合蛋白IHC检测,该类阳性的肺癌患者通常可从ALK抑制剂治疗中获益[7,27-28]。
适合ALK检测的肿瘤样本,包括肿瘤组织标本和细胞学标本。肿瘤标本获取手段包括手术切除、支气管镜检、经皮肺穿刺、淋巴结活检、手术活检等;对于恶性胸腔积液、心包积液、痰液或支气管灌洗液和细胞学穿刺等样本,恶性胸腔积液等细胞学样本在细胞数量充足条件下可制备细胞学样本蜡块,检测方法可采用IHC或RT-PCR或FISH;如果是新鲜细胞标本可考虑采用RT-PCR方法。考虑到细胞学样本的细胞数量少等特点,细胞学标本的检测结果解释需格外谨慎。检测实验室应根据组织标本类型选择合适的检测技术。当怀疑一种技术的可靠性时(如FISH的肿瘤细胞融合率接近15%时),可以考虑采用另一种技术加以验证。
目前,CFDA批准的ALK阳性NSCLC的诊断试剂盒有雅培贸易(上海)有限公司的ALK基因重组检测试剂盒(荧光原位杂交法)、罗氏诊断产品(上海)有限公司的Ventana anti-ALK抗体诊断试剂盒(免疫组织化学法)和厦门艾德生物医药科技有限公司的EML4-ALK融合基因检测试剂盒(荧光PCR法)。
ROS1阳性NSCLC与EGFR突变、ALK阳性NSCLC一样,是NSCLC的另一种特定分子亚型[31-32]。已有多个研究表明晚期ROS1阳性NSCLC克唑替尼治疗有效[33-35]。CFDA批准的ROS1阳性NSCLC的诊断试剂盒为厦门艾德生物医药科技有限公司的ROS1融合基因检测试剂盒(荧光PCR法)等。
近年,多项研究采用NGS针对晚期NSCLC进行多基因检测,如目前可作为治疗靶点的基因变异:EGFR敏感突变,EGFR T790M突变,KRAS突变,HER2突变,ALK融合基因,ROS1融合基因,BRAF V600E突变,RET融合基因,MET融合基因,MET-14外显子跳跃突变等。NGS的标本可为组织或外周血游离DNA。NGS的应用提高了临床检测效率,可更加精准的指导NSCLC的治疗。同时,NGS亦可用于发现未知基因,探索动态疗效检测、判断预后及发现耐药机制等[36-43]。但目前,由于成本高、检测市场不规范、检测效率和质量不能保证,中国市场仍无足够靶向治疗药物等因素限制了NGS的大规模临床应用。
多项研究提示,PD-L1蛋白表达和肿瘤突变负荷可能预测免疫检查点抑制剂治疗的疗效[44,45]。
五、基于病理类型、分期和分子分型的综合治疗
非小细胞肺癌的治疗
ⅠA、ⅠB期原发性非小细胞肺癌的治疗

【注释】
1.肺癌外科手术标准[5]
肺癌手术应做到完全性切除。
(1)完全性切除:
1)切缘阴性,包括支气管、动脉、静脉、支气管周围、肿瘤附近组织;
2)淋巴结至少6组,其中肺内3组;纵隔3组(必须包括7区);
3)切除的最高淋巴结镜下阴性;
4)淋巴结无结外侵犯。
(2)不完全性切除:
1)切缘肿瘤残留;
2)胸腔积液或心包积液癌细胞阳性;
3)淋巴结结外侵犯;
4)淋巴结阳性但不能切除。
(3)不确定切除:
切缘镜下阴性,但出现下列情况之一者:
1)淋巴结清扫未达要求;
2)切除的最高纵隔淋巴结阳性;
3)支气管切缘为原位癌;
4)胸腔冲洗液细胞学阳性。
2.辅助化疗
ⅠA期非小细胞不建议辅助化疗,ⅠB期非小细胞肺癌(包括有高危因素的肺癌),由于缺乏高级别证据的支持,一般不推荐辅助化疗(2A类证据)[1,2,18,19]。
3.先进放疗技术[1,7-10,14-17]
包括4D-CT和(或)PET/CT定位系统,VMAT(容积旋转调强放射治疗技术),IGRT(影像引导放射治疗),呼吸运动控制,质子治疗等。
4.不完全切除患者
二次手术±化疗(2A类证据)[1,2]或术后三维适形放疗±化疗[ⅠB期(2A类证据),ⅠA期(2B类证据)][1,2]。
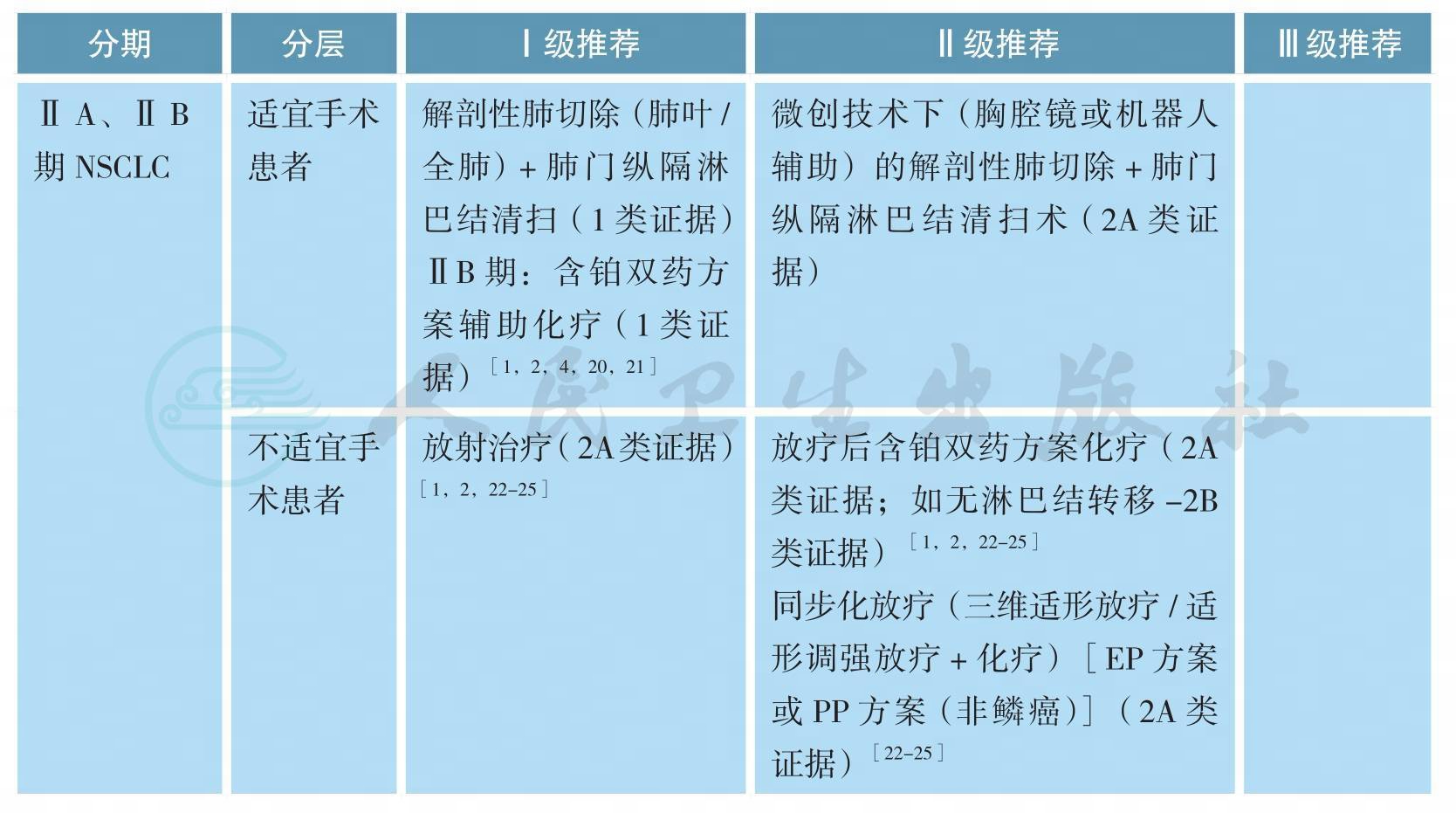
ⅡA、ⅡB期原发性非小细胞肺癌的治疗
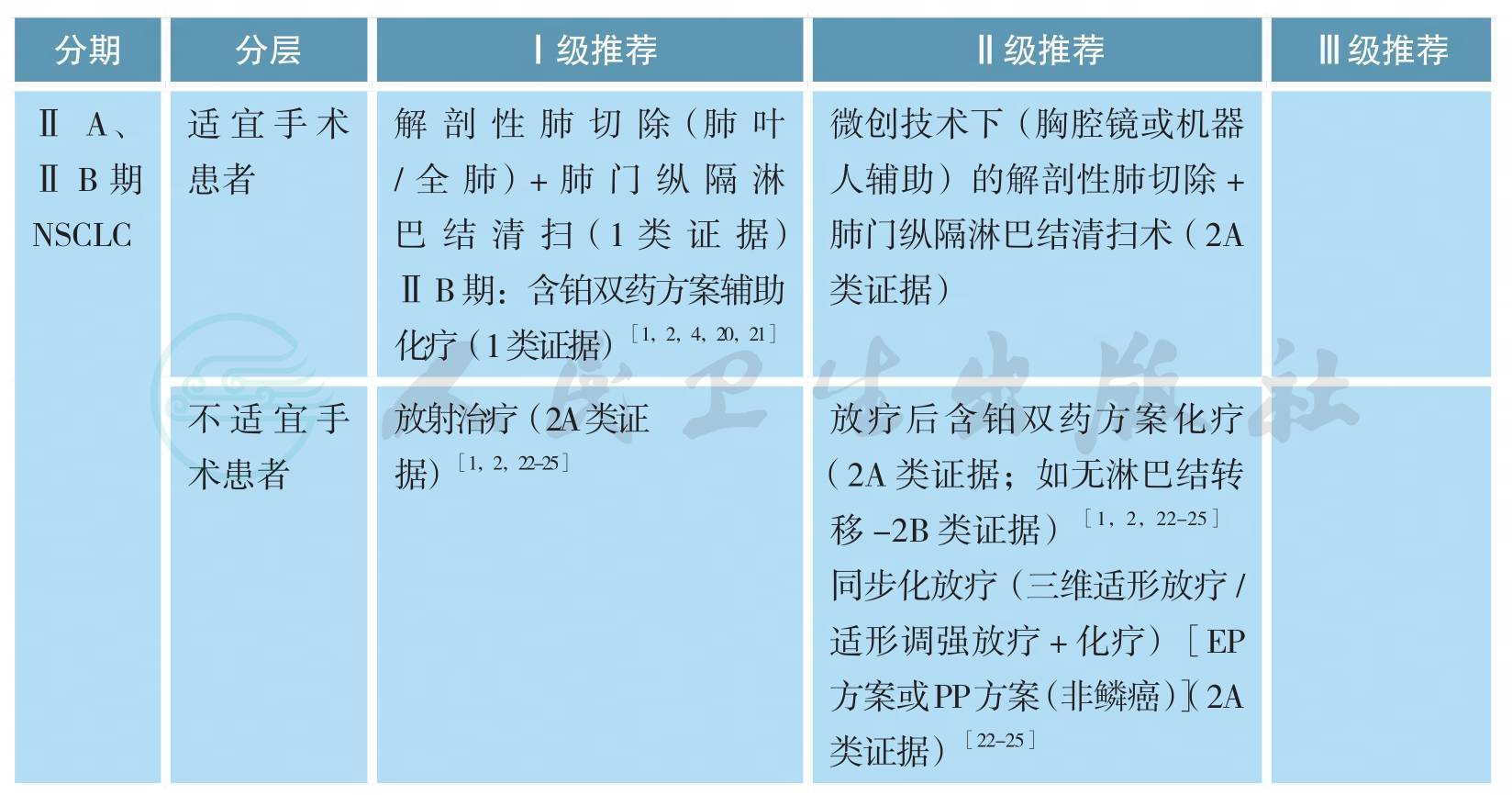
【注释】
可选辅助化疗方案包括:长春瑞滨/紫杉醇/多西他赛/培美曲塞(非鳞癌)/吉西他滨+顺铂/卡铂(2A类证据)[1-3,26]。
第八版分期中ⅡA期患者,由于缺乏高级别证据的支持,完全性切除后,一般不推荐辅助化疗(2A类证据)[1,2,18,19]。
不完全切除患者,行二次手术+含铂双药方案化疗(2A类证据)[1-3]或术后放疗+含铂双药方案化疗(2A类证据)[1-3]。
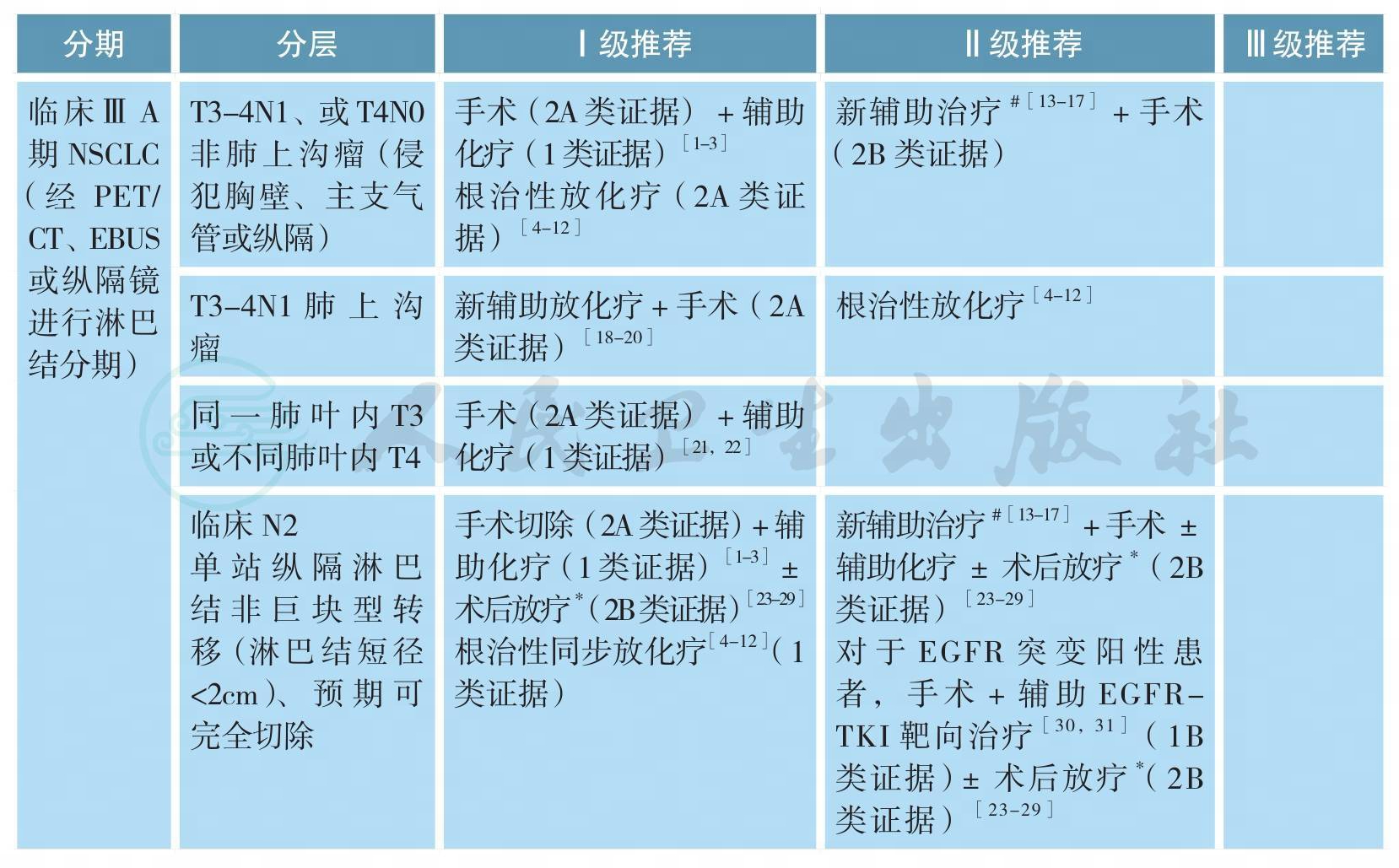
可手术ⅢA期原发性非小细胞肺癌的治疗
可手术ⅢA期原发性非小细胞肺癌的治疗(续)
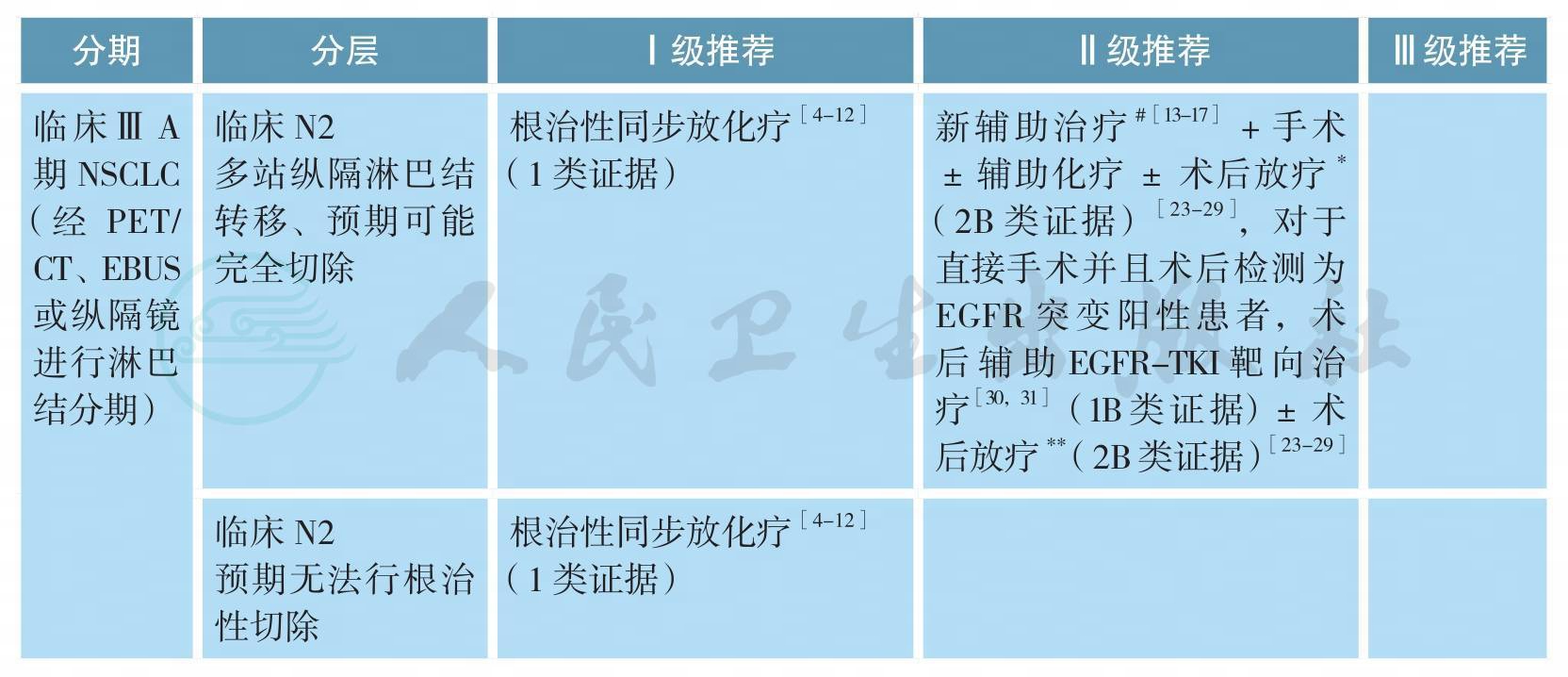
# 新辅助治疗模式包括:单纯化疗、序贯化放疗、同步放化疗、化疗后同步放化疗等,最佳模式尚未确定
[13-17]*术后病理N2可以考虑术后放疗(2B类证据)或加入术后放疗随机分组研究
[23-29]**该组患者的局部区域复发风险较单站N2淋巴结转移患者进一步升高,术后病理N2可以考虑术后放疗(2B类证据)
[23-29]【注释】
ⅢA期NSCLC是异质性很大的一组疾病。根据AJCC第7版分期,ⅢA期包括:T3N1、T4N0-1和T1-3N2。在治疗前完整分期检查的基础上,根据治疗前初评是否可行完全性切除,可将ⅢA期NSCLC分为如下三组:①可完全性手术切除,即R0切除;②可能完全性手术切除;③无法完全性切除。根据术后病理N分期,可将患者分为pN0-1和pN2两个亚组。
1.临床判断可完全性手术切除的ⅢA期NSCLC
包括T3N1、部分T4N1(如肿瘤直接侵犯胸壁、主支气管或纵隔)伴或不伴有单站纵隔淋巴结转移的病变。对于该组患者,推荐首先进行手术切除,术后辅助含铂双药方案化疗[1,2];若术后病理N分期为N0-1,不需进行术后放疗[3];若病理分期为N2,是否需进行术后放疗尚存争议,详见病理N2期NSCLC的术后放疗。另一基本策略为根治性同步放化疗,详见ⅢB期NSCLC的治疗[4-12]。可选策略为新辅助治疗后再行根治性切除(详见ⅢA期NSCLC的新辅助治疗)[13-17]。
2.局部侵犯胸壁但无纵隔淋巴结转移(T3N1)的肺上沟瘤
目前推荐治疗为新辅助同步放化疗后进行完全性手术切除[18-20],2年生存率为50%~70%,5年生存率为40%。对于不能直接进行R0切除的ⅢA期NSCLC,基本策略为根治性同步放化疗(详见ⅢB期NSCLC的治疗)[4-12]。可选策略为新辅助治疗后(详见ⅢA期NSCLC的新辅助治疗),再评估,决定给予完全性切除或是继续放化疗至根治剂量[13-17]。目前尚无高级别证据显示新辅助化疗后联合手术能够优于根治性放化疗,也无证据表明新辅助放化疗+手术的三联疗法能够优于化疗+手术或根治性放化疗的二联疗法。
对于同一肺叶内多个病灶的T3病变和同侧肺不同肺叶内多个病灶的T4病变,推荐治疗为肺叶切除或全肺切除术后辅助化疗[21,22]。对于术后病理分期N0-1的患者,不推荐术后放疗;对于术后N2患者,除辅助化疗外(2A类证据),是否需进行术后放疗尚存争议(详见病理N2期NSCLC的术后放疗)[23-29]。
3.无法进行完全性切除的病变
如肿瘤局部侵犯很广、预计新辅助治疗后仍无法达到R0切除、多站纵隔淋巴结转移,首选治疗方式为根治性放化疗(1类证据)[4-12],目前尚无证据支持后续巩固化疗[32-35],详见ⅢB期NSCLC的治疗。同步化疗方案主要包括:顺铂+依托泊苷;卡铂+紫杉醇或顺铂/卡铂+培美曲塞[5,36-38]。同步化疗首选推荐方案为顺铂+依托泊苷[39,40];放疗推荐剂量为60~70Gy,目前尚无证据表明提高局部放疗剂量能够改善疗效[41]。2017年发表的PACIFIC研究是一项针对不可手术切除的局部晚期NSCLC根治性同步放化疗后巩固PD-L1抑制剂Durvalumab对比安慰剂的Ⅲ期随机对照研究。结果显示同步放化疗后Durvalumab巩固治疗组的PFS显著优于安慰剂组,中位PFS分别为16.8个月和5.6个月(HR=0.52,P<0.001)。而且,Durvalumab巩固治疗组的疾病缓解率显著高于对照组、疾病缓解维持时间、发生远处转移或死亡的时间均得以显著延长[42]。在最近发布的NCCN 2018年第1版中,Durvalumab已被推荐作为局部晚期NSCLC同步放化疗后巩固治疗。基于PACIFIC研究结果,Durvalumab于2018年2月在美国获批上市。
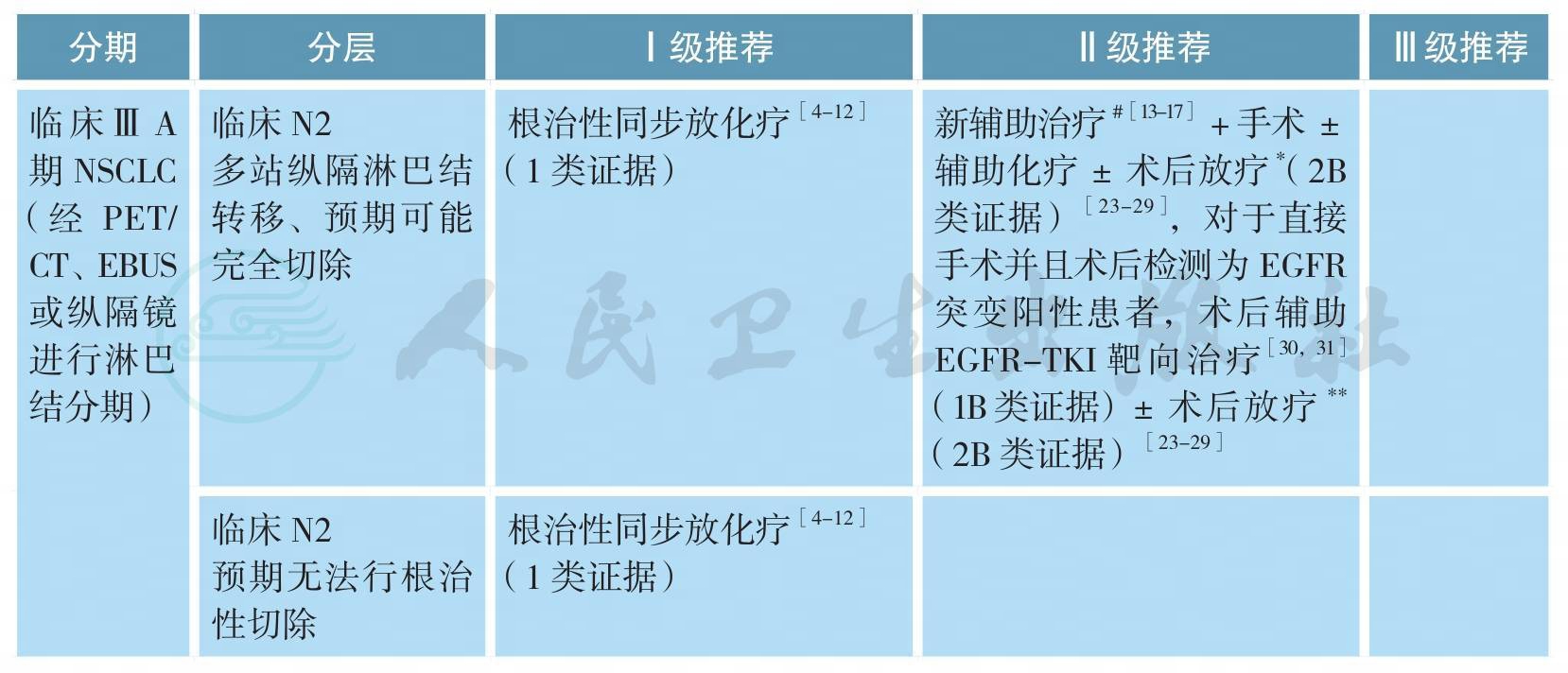
4.ⅢA期NSCLC的新辅助治疗
对于部分ⅢA/N2期非小细胞肺癌(NSCLC),已有多项探讨各种新辅助治疗联合手术模式对比传统根治性放化疗的随机对照研究。迄今为止,前期发表的联合治疗模式包括:诱导化疗后手术对比放疗(EORTC 08941,ⅢA/N2新辅助化疗3周期后随机接受手术vs.根治性放疗)、诱导放化疗后手术对比根治性放化疗(INT0139,pN2患者,新辅助同步放化疗后接受手术vs根治性同步放化疗,并都辅以2个周期巩固化疗)、新辅助化疗后手术对比新辅助序贯放化疗后手术(SAKK,ⅢA/N2新辅助化疗3个周期后根治性手术vs.新辅助诱导化疗续贯放疗44Gy/22次后根治性手术)和新辅助化疗+序贯同步放化疗后根治性手术对比新辅助化疗后续贯根治性放化疗(ESPATUE,ⅢA/N2期和部分选择性ⅢB,3个周期的PC方案新辅助化疗后同步放化疗,45Gy/1.5Gy,Bid/3周,同步1个周期顺铂+长春瑞滨,可切除病变接受推量至根治性放化疗vs.根治性手术)[13-17]。
EORTC08941研究入组了579例ⅢA期NSCLC患者,在接受了3个周期诱导化疗后达到CR/PR的322例患者被随机分配进入手术切除或放射治疗。结果显示,两组的OS(16.4个月vs.17.5个月,P=0.596)和PFS(9.0个月vs.11.3个月,P=0.605)无统计学差异[13]。INT 0139研究入组了429例ⅢA期NSCLC,所有患者接受了EP方案的同步放化疗(45Gy/25次)后,随机分配进入手术组或根治性放疗组,两组患者后续都进行2个周期的巩固化疗。结果显示两组的OS相仿(23.6个月vs.22.2个月,P=0.24);手术组具有一定的PFS优势(12.8个月vs.10.5个月,P=0.017);亚组分析显示新辅助同步放化疗后接受肺叶切除的患者可能具有一定的OS优势(33.6个月vs.21.7个月,P=0.002)[14]。SAKK研究纳入了2001—2012年23个中心的232例T1-3N2的ⅢA/N2期非小细胞肺癌患者,随机分为诱导化疗组和诱导序贯放化疗组,并以研究中心、体重减轻(>5%)和纵隔大肿块(直径≥5cm)进行分层随机。全组中位随访时间52.4个月,诱导放化疗组和诱导化疗组接受手术切除的患者比例分别为85%和82%,诱导治疗有效率分别为61%和44%,手术完全切除率分别为91%和81%(P=0.06);但两组的病理完全缓解率和淋巴结降期率相似,术后并发症亦无差别。诱导放化疗或诱导化疗的两组患者的无病生存期(12.8个月vs.11.6个月,P=0.67)及总生存期(37.1个月vs.26.2个月)无明显统计学差异,两组整体失败模式无区别[15]。ESPATUE研究包括ⅢA/N2期和部分选择性ⅢB期NSCLC患者。所有患者接受3个周期的PC方案新辅助化疗后给予同步放化疗(45Gy/1.5Gy,Bid/3周,同步1个周期顺铂+长春瑞滨化疗)后经多学科讨论评估病变手术切除性,可手术切除的患者被随机分组到同步放化疗组(放疗加量20~26Gy组)和手术组。研究拟入组500例患者,但因入组缓慢而提前关闭,关闭时共入组246例患者,最终80例患者进入放疗加量组,81例患者进入手术组。研究结果显示,放疗组和手术组的5年OS分别为40%和44%(P=0.34),PFS分别为35%和32%(P=0.75),其中手术组术后pCR率为33%[16]。GLCCG研究入组了558例ⅢA和ⅢB期(ⅢB其中超过40%的患者为T4N1病变,实际为目前的ⅢA期)NSCLC,患者被随机分配到新辅助化疗+手术+放疗vs.新辅助化疗+同步放化疗+手术两个治疗组。结果显示,两组的PFS(9.5个月vs.10.0个月,P=0.87)和OS(15.7个月vs.17.6个月,P=0.97)都没有区别[17]。
综上所述,除了INT0139研究显示手术组有PFS优势,亚组分析显示新辅助同步放化疗后接受肺叶切除的患者可能具有一定的OS优势外,其他研究皆未能显示出研究组和对照组在生存方面的优势。所以,基于现有研究证据,对于ⅢA期NSCLC,根治性同步放化疗作为主要治疗模式的地位仍未动摇,对于可手术患者新辅助治疗联合手术可作为治疗选择之一,但新辅助治疗模式(单纯化疗、序贯化放疗、同步放化疗、化疗后同步放化疗)仍待进一步研究。
5.病理N2期NSCLC的术后放疗
以三维适形和调强放疗为代表的精确放疗技术广泛应用于肺癌的治疗,进一步降低了心脏毒性等放射损伤导致的非肿瘤病死率。迄今为止,已有多项多中心大样本回顾性研究评估了3DCRT/IMRT技术条件下Ⅲ-N2非小细胞肺癌术后放射治疗(PORT)的价值。
Corso等对美国国家癌症数据库(NCDB)1998—2006年间Ⅱ~Ⅲ期R0切除的NSCLC进行回顾性病例对照研究,其中pN2期患者6979例,结果显示PORT组和对照组5年总生存率分别为34.1%和27.8%(P<0.001),PORT使生存率绝对值提高了6.3%[23]。Urban等对SEER数据库1998—2009年手术切除的4773例pN2患者的分析显示,PORT组的死亡风险显著降低(HR=0.9,P=0.026),结论与上述研究一致[24]。在辅助化疗已经成为淋巴结转移NSCLC完全性切除术后标准治疗的前提下,Mikell等针对NCDB数据库2004~2006年间接受化疗的2115例pN2患者进行PORT的作用分析,结果PORT显著改善了患者的总生存,两组中位生存期分别为42个月和38个月,5年OS分别为39.8%和34.7%(P=0.048),多因素分析也显示PORT是显著改善生存的独立预后因素(HR=0.87,P=0.026)[25]。Robinson等对NCDB数据库2006~2010年间接受化疗的4483例pN2期NSCLC进行分析,结果同样显示PORT显著提高了中位生存(45.2个月vs.40.7个月)和5年OS(39.3%vs.34.8%,P=0.014),而且多因素分析显示PORT是独立的预后因素(HR=0.888,P=0.029)[26]。
上述研究结果均显示PORT可能改善Ⅲ-N2期NSCLC患者的总生存。但是老年患者因为合并症多、对放疗耐受性差,接受PORT是否也能同样获益还需要进一步的研究。Wisnivesky等对1992~2005年SEER数据库中≥65岁、接受根治性切除的pN2期NSCLC患者进行分析,其中术后放疗组710例,对照组597例,PORT与对照组相比年龄更小、经济情况更好,其他临床特性两组具有可比性。结果PORT未能改善老年患者的总生存,HR为1.11(P=0.30),作者建议对N2期NSCLC开展PORT的随机分组研究[27]。
目前国内外针对完全切除术加辅助化疗后的ⅢA-N2患者采用3DCRT/IMRT的随机分组研究主要有三组。美国1998—2000年开展了CALGB 9734随机分组研究,入组条件为完全性切除的pⅢA-N2非小细胞肺癌,术后接受2~4周期PC方案辅助化疗后,随机分入PORT组和观察组,放疗采用3DCRT技术,50Gy/25次。预期入组480例患者,但是实际上仅完成37例,放疗组和对照组患者1年的生存率(74%vs.72%)和无复发生存率均无显著性差异,研究因入组缓慢而失败[28]。欧洲自2007年启动了大规模的随机对照Ⅲ期临床研究(Lung ART),研究采用三维适形放疗技术,预计样本量为700例,预期到2017年完成入组,然而到目前为止尚未看到该研究的后继报道[29]。中国医学科学院肿瘤医院放疗科牵头组织和启动了“N2(ⅢA期)非小细胞肺癌术后化疗后三维精确放射治疗多中心随机对照Ⅲ期临床研究”,研究针对完全性切除ⅢA-N2非小细胞肺癌患者,术后进行4个周期的含铂方案化疗,辅助化疗结束后进行全面复查,未出现肿瘤复发者随即进入PORT组和观察组。研究预计入组500例,目前已经完成近400例。
目前术后放疗推荐采用三维适形或调强技术,靶区主要包括同侧肺门(残端)、同侧纵隔和隆突下等局部区域复发的高危区域,总剂量50~54Gy。
6.EGFR突变阳性患者术后辅助治疗
EGFR-TKI辅助治疗一直都在探索过程中。BR.19以及RADIANT研究均探索了TKI在ⅠB-ⅢA期、EGFR非选择NSCLC人群中的术后辅助治疗价值,但均以失败告终,显示相比与安慰剂相比,辅助TKI并未能进一步改善DFS [43,44]。然而在RADIANT研究中161例(16.5%)EGFR突变阳性患者亚组分析显示,厄洛替尼组DFS更长(46.4个月vs.28.5个月,HR=0.61),但未达统计学差异[44]。CTONG1104(ADJUVANT)研究是首个在EGFR突变阳性、完全切除的病理Ⅱ~ⅢA期(N1-N2)的NSCLC患者中,比较了吉非替尼对比长春瑞滨+顺铂方案的前瞻性随机、对照Ⅲ期临床试验,共入组222例患者。与化疗相比,吉非替尼显著延长了中位DFS(18.0个月vs.28.7个月,HR=0.60,P=0.0054),亚组分析显示,N2患者从术后辅助靶向治疗中获益更多[30]。另有一项2017年在世界肺癌大会上报告的厄洛替尼对比化疗作为完全切除术后、伴有EGFR突变的ⅢA期NSCLC患者的辅助治疗的疗效与安全性的Ⅱ期临床研究(EVAN研究),结果显示与化疗相比,厄洛替尼显著提高2年DFS率(44.62%vs.81.35%,P<0.001),中位DFS尚未成熟[31]。关于EGFR突变阳性病人术后TKI的用药时间,现有研究多采用2年维持治疗[30,31,43,44],尚无随机对照研究证据提供最佳的维持用药时间。对于EGFR突变阳性且接受TKI辅助治疗的ⅢA期NSCLC,术后辅助放疗的作用和时机尚无不明确。
新药在美国及欧盟获批适应证(截至2018.03)

不可手术ⅢA、ⅢB期原发性非小细胞肺癌的治疗
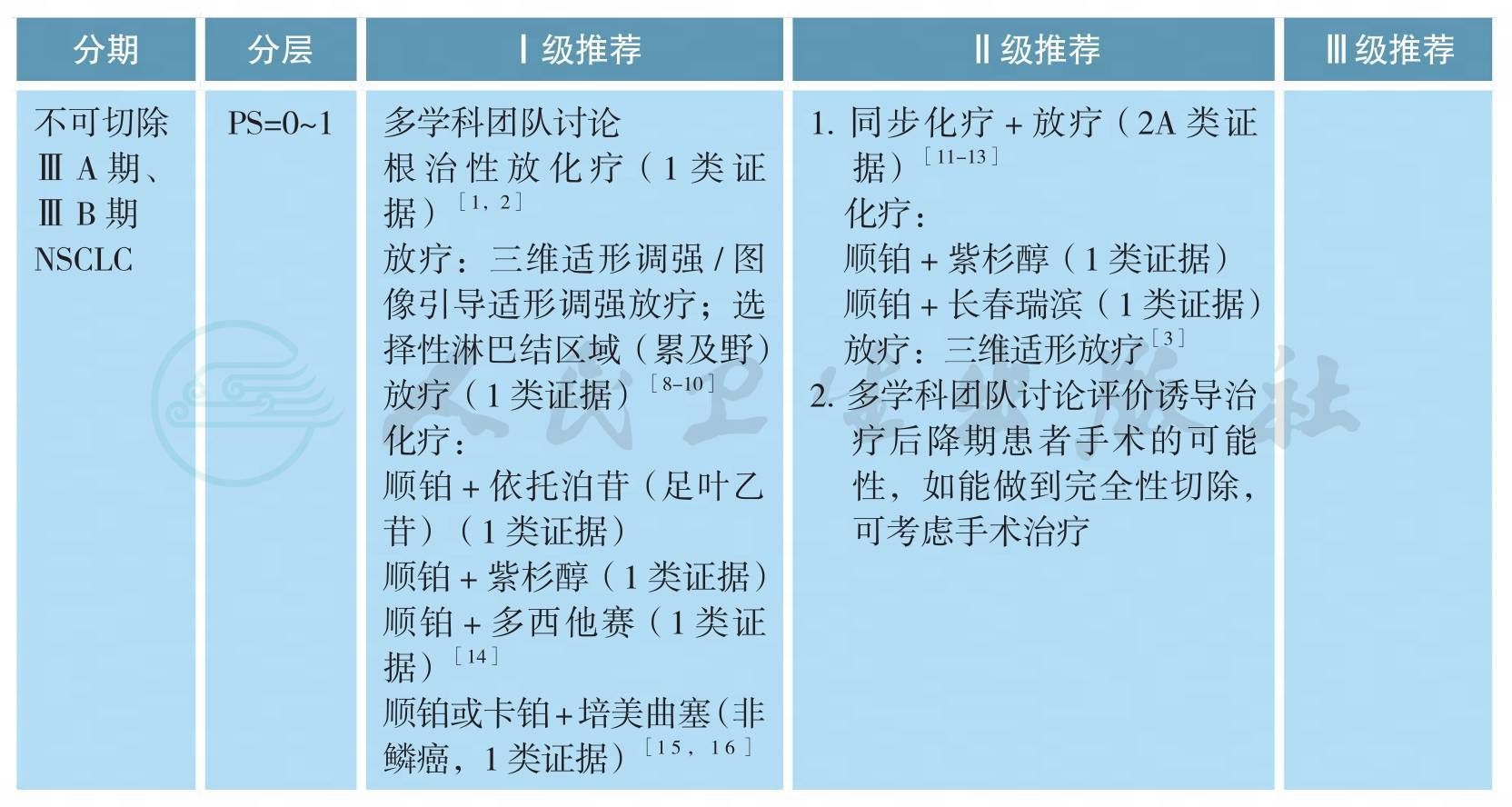
不可手术ⅢA、ⅢB期原发性非小细胞肺癌的治疗(续)

备注:
不可切除ⅢA期、ⅢB期主要指有如下影像或淋巴结病理性证据:
1.同侧纵隔淋巴结多枚转移成巨大肿块或多站转移(ⅢA:T1-3N2或ⅢB:T4N2)。
2.对侧肺门、纵隔淋巴结,或同、对侧斜角肌或锁骨上淋巴结转移(ⅢB:T1-4N3)。
3.病灶侵犯心脏、主动脉和食管(ⅢB:T4N0-1)。
同步放化疗方案:
EP:顺铂 50mg/m2,d1,8,29,36;依托泊苷 50mg/m2,d1~5,d29~30;
PC:卡铂AUC 2,紫杉醇45~50mg/m2,每周;
AP:顺铂75mg/m2,d1;培美曲塞500mg/m2,d1,每3周重复(非鳞癌);
AC:卡铂AUC 5,d1;培美曲塞500mg/m2,d1,每3周重复(非鳞癌)。
放疗方案:60~66Gy/30~33次/6~7周。
【注释】
本节指南中,有根治性治疗指征的患者,并PS评分良好,放疗计划剂量参数符合剂量学要求,推荐同步放化疗[1,2]。如采用PC周剂量化疗2个周期同步放疗,随后2个周期化疗应实施3周方案。放疗技术标准至少要求基于CT定位的三维适形放疗(3D-CRT)[3],放疗方案推荐采用常规分割,靶区剂量60~66Gy/30~33次/6~7周。RTOG 0617研究表
二、影像和分期诊断
1.National Lung Screening Trial Research Team,Aberle DR,Berg CD,et al.The National Lung Screening Trial:overview and study design.Radiology,2011,258(1):243-253.
2.National Lung Screening Trial Research Team,Aberle DR,Adams AM,et al.Reduced lung-cancer mortality with low-dose computed tomographic screening.N Engl J Med,2011,365(5):395-409.
3.National Lung Screening Trial Research Team,Aberle DR,Adams AM,et al.Baseline characteristics of participants in the randomized national lung screening trial.J Natl Cancer Inst,2010,102(23):1771-1779.
4.NCCN Clinical Practice Guidelines in Oncology(NCCN Guidelines®)Non-Small Cell Lung Cancer(Version 2,2018).
5.Wu Y,Li P,Zhang H,et al.Diagnostic value of fluorine 18 fluorodeoxyglucose positron emission tomography/computed tomography for the detection of metastases in non-small-cell lung cancer patients.Int J Cancer,2013,132(2):E37-E47.
6.Carney DN.Lung cancer--time to move on from chemotherapy.N Engl J Med,2002,346(2):126-128.
7.Chute JP,Chen T,Feigal E,et al.Twenty years of phase Ⅲ trials for patients with extensive-stage small-cell lung cancer:perceptible progress.J Clin Oncol,1999,17(6):1794-1801.
三、病理学诊断
1.Travis WD,Brambilla E,Nicholson AG,et al.The 2015 World Health Organization classification of lung tumors:impact of genetic,clinical and radiologic advances since the 2004 classification.J Thorac Oncol,2015,10(9):1243-1260.
2.Travis WD,Brambilla E,Burke A,et al.WHO classification of tumours of the lung,pleura,thymus and heart.2015:International Agency for Research on Cancer.
3.Rekhtman N,Ang DC,Sima CS,et al.Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens.Mod Pathol,2011,24(10):1348-1359.
4.Nonaka D.A study of DeltaNp63 expression in lung non-small cell carcinomas.Am J Surg Pathol,2012,36(6):895-899.
5.Cunha SG,Saieg MA.Cell blocks for subtyping and molecular studies in non-small cell lung carcinoma.Cytopathology,2015,26(5):331-333.
6.Kapila K,Al-Ayadhy B,Francis IM,et al.Subclassification of pulmonary non-small cell lung carcinoma in fine needle aspirates using a limited immunohistochemistry panel.J Cytol,2013,30(4):223-225.
7.Kimbrell HZ,Gustafson KS,Huang M,et al.Subclassification of non-small cell lung cancer by cytologic sampling:a logical approach with selective use of immunocytochemistry.Acta Cytol,2012,56(4):419-424.
8.Treece AL,Montgomery ND,Patel NM,et al.FNA smears as a potential source of DNA for targeted next-generation sequencing of lung adenocarcinomas.Cancer Cytopathol,2016,124(6):406-414.
9.Betz BL,Dixon CA,Weigelin HC,et al.The use of stained cytologic direct smears for ALK gene rearrangement analysis of lung adenocarcinoma.Cancer Cytopathol,2013,121(9):489-499.
10.Hasleton P,Flieder DB.Spencer's Pathology of the Lung.6th ed.Cambridge University Press,2013.
11.Butnor KJ,Beasley MB,Cagle PT,et al.Protocol for the examination of specimens from patients with primary non-small cell carcinoma,small cell carcinoma,or carcinoid tumor of the lung.Arch Pathol Lab Med,2009,133(10):1552-1559.
12.Travis WD,Brambilla E,Rami-Porta R,et al.Visceral pleural invasion:pathologic criteria and use of elastic stains:proposal for the 7th edition of the TNM classification for lung cancer.J Thorac Oncol,2008,3(12):1384-1390.
13.Leighl NB,Rekhtman N,Biermann WA,et al.Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors:American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the study of lung cancer/association for molecular pathologyguideline.J Clin Oncol,2014,32(32):3673-3679.
四、分子分型
1.Zhong WZ,Wang Q,Mao WM,et al.Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stageⅡ -ⅢA(N1-N2)EGFR-mutant NSCLC(ADJUVANT/CTONG1104):A randomised,open-label,phase 3 study.Lancet Oncol,2018,19(1):139-148.
2.Yue D,Xu S,Wang Q,et al.Efficacy and Safety of Erlotinib vs Vinorelbine/Cisplatin as Adjuvant Therapy for Stage ⅢA EGFR Mutant NSCLC Patients(EVAN,NCT01683175).WCLC 2017,OA 16.04.
3.Mok TS,Wu YL,Thongprasert S,et al.Gefitinib or carboplatin-paclitaxel in pulmonaryadenocarcinoma.N Engl J Med,2009,361(10):947-957.
4.Zhou C,Wu YL,Chen G,et al.Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer(OPTIMAL,CTONG-0802):a multicentre,open-label,randomised,phase 3 study.Lancet Oncol,2011,12(8):735-742.
5.Wu YL,Zhou C,Liam CK,et al.First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer:analyses from the phase Ⅲ,randomized,open-label,ENSURE study.Ann Oncol,2015,26(9):1883-1889.
6.Wu YL,Zhou C,Hu CP,et al.Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations(LUX-Lung 6):an open-label,randomised phase 3 trial.Lancet Oncol,2014,15(2):213-222.
7.Solomon BJ,Mok T,Kim DW,et al.First-line crizotinib versus chemotherapy in ALK-positive lung cancer.N Engl J Med,2014,371(23):2167-2177.
8.Kris MG,Johnson BE,Berry LD,et al.Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs.JAMA,2014,311(19):1998-2006.
9.Sacher AG,Dahlberg SE,Heng J,et al.Association between younger age and targetable genomic alterations and prognosis in non-small-cell lung cancer.JAMA Oncol,2016,2(3):313-320.
10.Barlesi F,Mazieres J,Merlio JP,et al.Routine molecular profiling of patients with advanced nonsmall-cell lung cancer:results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup(IFCT).Lancet,2016,387(10026):1415-1426.
11.Pao W,Girard N.New driver mutations in non-small-cell lung cancer.Lancet Oncol,2011,12(2):175-180.
12.Gerber DE,Gandhi L,Costa D,et al.Management and future directions in non-small cell lung cancer with known activating mutations.ASCO Education Book,2014,16:e353-e365.
13.Travis WD,Brambilla E,Nicholson AG,et al.The 2015 World Health Organization classification of lung tumors:impact of genetic,clinical and radiologic advances since the 2004 classification.J Thorac Oncol,2015,10(9):1243-1260.
14.Wu YL,Zhong WZ,Li LY,et al.Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer:a meta-analysis based on updated individual patient data from six medical centers in mainland China.J Thorac Oncol,2007,2(5):430-439.
15.Shi Y,Au JS,Thongprasert SA,et al.Prospective,molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology(PIONEER).J Thorac Oncol,2014,9(2):154-162.
16.Gou LY,Wu YL.Prevalence of driver mutations in non-small-cell lung cancers in the People’S Republic of China.Lung Cancer Target Therapy,2014,5:1-9.
17.Yang JCH,Sequist LV,Greater SL,et al.Clinical activity of afatinib in patients with advanced nonsmall-cell lung cancer harbouring uncommon EGFR mutations:a combined post-hoc analysis of LUXLung 2,LUX-Lung 3,and LUX-Lung 6.Lancet Oncol,2015,16(7):830-838.
18.Goto K,Ichinose Y,Ohe Y,et al.Epidermal growth factor receptor mutation status in circulating free DNA in serum:from IPASS,a phase Ⅲ study of gefitinib or carboplatin/paclitaxel in non-small cell lung cancer.J Thorac Oncol,2012,7(1):115-121.
19.Bai H,Mao L,Wang HS,et al.Epidermal growth factor receptor mutations in plasma DNA samples predict tumor response in Chinese patients with stages Ⅲ B to Ⅳ non-small-cell lung cancer.J Clin Oncol,2009,27(16):2653-2659.
20.Douillard JY,Ostoros G,Cobo M,et al.Gefitinib treatment in EGFR mutated caucasian NSCLC circulating-free tumor DNA as a surrogate for determination of EGFR status.J Thorac Oncol,2014,9(9):1345-1353.
21.Mok T,Wu YL,Lee JS,et al.Detection and dynamic changes of EGFR mutation from circulating tumor DNA as a predictor of survival outcome in NSCLC patients treated with erlotinib and chemotherapy.Clin Cancer Res,2015,21(14):3196-3203.
22.Su KY,Chen HY,Li KC,et al.Pretreatment epidermal growth factor receptor(EGFR)T790M mutation predicts shorter EGFR tyrosine kinase inhibitor response duration in patients with non-smallcell lung cancer.J Clin Oncol,2012,30(4):433-440.
23.Ahn M,Han J,Tsai C.et al.Detection of EGFR mutations from plasma ctDNA in the osimertinib PhaseⅢ trial(AURA3):comparison of three plasma assays.WCLC,2017,OA 10.01.
24.Wang ZJ,Chen R,Wang SH,et al.Quantification and Dynamic Monitoring of EGFR T790M in Plasma Cell-Free DNA by Digital PCR for Prognosis of EGFR-TKI Treatment in Advanced NSCLC.PLoS One.2014;9(11):e110780.
25.Wang J,Cheng Y,Wu YL,et al.Gefitinib as First-Line Treatment of Plasma CtDNA EGFR Mutation-Positive NSCLC Detected by DdPCR:BENEFIT Study(CTONG1405),WCLC,2017,MA 11.03.
26.Gray J,Okamoto I,Sriuranpong V,et al.Osimertinib vs SoC EGFR-TKI as First-Line Treatment in Patients with EGFRm Advanced NSCLC(FLAURA):Plasma ctDNA Analysis,WCLC,2017,OA 05.02.
27.Shaw AT,Yeap BY,Mino-Kenudson M,et al.Clinical features and outcome of patients with nonsmall-cell lung cancer who harbor EML4-ALK.J Clin Oncol,2009,27(26):4247-4453.
28.Kwak EL,Bang YJ,Camidge DR,et al.Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer.N Engl J Med,2010 Oct 28,363(18):1693-1703.
29.Zhang XC,Zhang S,Yang XN,et al.Fusion of EML4 and ALK is associated with development of lung adenocarcinomas lacking EGFR and KRAS mutations and is correlated with ALK expression.Mol Cancer,2010,9:188.
30.Hong S,Fang W,Hu Z,et al.A large-scale cross-sectional study of ALK rearrangements and EGFR mutations in non-small-cell lung cancer in Chinese Han population.Sci Rep,2014,4:726-728.
31.Cai W,Li X,Su C,et al.ROS1 fusions in Chinese patients with non-small-cell lung cancer.Ann Oncol,2013,24(7):1822-1827.
32.Bergethon K,Shaw AT,Ou SH,et al.ROS1 rearrangements define a unique molecular class of lung cancers.J Clin Oncol,2012,30(8):863-870.
33.Shaw AT,Ou SH,Bang YJ,et al.Crizotinib in ROS1-rearranged non-small-cell lung cancer.N Engl J Med,2014,371(21):1963-1971.
34.Mazi ères J,Zalcman G,Crinò L,et al.Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement:results from the EUROS1 cohort.J Clin Oncol,2015,33(9):992-999.
35.Goto K,Yang JCH,Kim DW,Wu YL,et al.Phase II study of crizotinib in east Asian patients(pts)with ROS1-positive advanced non-small cell lung cancer(NSCLC).2016 American Society of Clinical Oncology(ASCO)Annual Meeting,J Clin Oncol 34,2016(suppl;abstr 9022).
36.Aziz N,Zhao Q,Bry L,et al.College of American Pathologists' laboratory standards for next-generation sequencing clinical tests.Arch Pathol Lab Med 2015;139:481-493.
37.Luthra R,Chen H,Roy-Chowdhuri S,Singh RR.Next-Generation Sequencing in Clinical Molecular Diagnostics of Cancer:Advantages and Challenges.Cancers(Basel)2015;7:2023-2036.
38.Drilon A,Wang L,Arcila ME,et al.Broad,hybrid capture-based next-generation sequencing identifies actionable genomic alterations in lung adenocarcinomas otherwise negative for such alterations by other genomic testing approaches.Clin Cancer Res 2015;21:3631-3639.
39.Robson ME,Bradbury AR,Arun B,et al.American Society of Clinical Oncology Policy Statement Update:Genetic and Genomic Testing for Cancer Susceptibility.J Clin Oncol 2015;33:3660-3667.
40.Yu PP,Vose JM,Hayes DF.Genetic cancer susceptibility testing:increased technology,increased complexity.J Clin Oncol 2015;33:3533-3534.
41.Cardarella S,Ortiz TM,Joshi VA,et al.The introduction of systematic genomic testing for patients with non-small-cell lung cancer.J Thorac Oncol 2012;7:1767-1774.
42.Li T,Kung HJ,Mack PC,Gandara DR.Genotyping and genomic profiling of non-small-cell lung cancer:implications for current and future therapies.J Clin Oncol 2013;31:1039-1049.
43.Planchard D.Identification of driver mutations in lung cancer:first step in personalized cancer.Target Oncol 2013;8:3-14.
44.Reck M,Rodriguez-Abreu D,Robinson AG,et al.Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer,N Engl J Med 2016;375:1823-33.
45.Herbst RS,Baas P,Kim DW,et al.Pembrolizumab versus docetaxel for previously treated,PD-L1-positive,advanced non-small-cell lung cancer(KEYNOTE-010):a randomised controlled trial,Lancet 2016;387:1540-50.
ⅡA、ⅡB期原发性非小细胞肺癌的治疗:
1.NCCN Clinical Practice Guidelines in Oncology(NCCN Guidelines®)Non-Small Cell Lung Cancer(Version 4,2016).
2.Vansteenkiste J,Crinò L,Dooms C,et al.2nd ESMO Consensus Conference on Lung Cancer:earlystage non-small-cell lung cancer consensus on diagnosis,treatment and follow-up.Ann Oncol,2014,25(8):1462-1474.
3.Howington JA,Blum MG,Chang AC,et al.Treatment of stage ⅠAnd Ⅱnon-small cell lung cancer.Diagnosis and management of of lung cancer,3rd.American College of Chest Physicians evidence-based clinical practice guidelines.Chest,2013,143(5 Suppl):e278S-e313S.
4.Darling GE,Allen MS,Decker PA,et al.Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1(less than hilar)non-small cell carcinoma:results of the American College of Surgery Oncology Group Z0030 Trial.J Thorac Cardiovasc Surg,2011,141(3):662-670.
5.Rami-Porta R,Wittekind C,Goldstraw P.International Association for the Study of Lung Cancer(IASLC)Staging Committee.Complete resection in lung cancer surgery:proposed definition.Lung Cancer,2005,49(1):25-33.
6.Chang JY,Senan S,Paul MA,et al.Stereotactic ablative radiotherapy versus lobectomy for operable stage Ⅰ non-small-cell lung cancer:a pooled analysis of two randomised trials. Lancet Oncol,2015,16(6):630-637.
7.Onishi H,Shirato H,Nagata Y,et al.Stereotactic body radiotherapy(SBRT)for operable stage Ⅰ nonsmall-cell lung cancer:can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys,2011,81(5):1352-1358.
8.Grills IS,Mangona VS,Welsh R,et al.Outcomes after stereotactic lung radiotherapy or wedge resection for stage Ⅰ non-small-cell lung cancer.J Clin Oncol,2010,28(6):928-935.
9.Crabtree TD,Denlinger CE,Meyers BF,et al.Stereotactic body radiation therapy versus surgical resection for stage Ⅰ non-small cell lung cancer.J Thorac Cardiovasc Surg,2010.140(2):377-386.
10.Timmerman R,Paulus R,Galvin J,et al.Stereotactic body radiation therapy for Inoperable early stage lung cancer.JAMA,2010,303(11):1070-1076.
11.Dai C,Shen J,Ren Y,et al.Choice of Surgical Procedure for Patients With Non-Small-Cell Lung Cancer≤1 cm or > 1 to 2cm Among Lobectomy,Segmentectomy,and Wedge Resection:A Population-Based Study.J Clin Oncol.2016 Sep 10;34(26):3175-82.
12.Khullar OV,Liu Y,Gillespie T,Higgins KA,et al.Survival After Sublobar Resection versus Lobectomy for Clinical Stage IA Lung Cancer:An Analysis from the National Cancer Data Base.J Thorac Oncol.2015 Nov;10(11):1625-33.
13.Nishio W,Yoshimura M,Maniwa Y,et al.Re-Assessment of Intentional Extended Segmentectomy for Clinical T1aN0 Non-Small Cell Lung Cancer.Ann Thorac Surg. 2016 Nov;102(5):1702-1710.
14.Baumann P,Nyman J,Hoyer M,et al.Outcome in a prospective phase Ⅱ trial of medically inoperable stage Ⅰ non- small-cell lung cancer patients treated with stereotactic body radiotherapy.J Clin Oncol,2009,27(20):3290-3296.
15.Grutters JPC,Kessels AGH,Pijls-Johannesma M,et al.Comparison of the effectiveness of radiotherapy with photons,protons and carbon-ions for non-small cell lung cancer:a meta-analysis.Radiother Oncol,2010,95(1):32-40.
16.Palma D,Visser O,Lagerwaard FJ,et al.Impact of introducing stereotactic lung radiotherapy for elderly patients with stage Ⅰ non-small-cell lung cancer:a population-based time-trend analysis.J Clin Oncol,2010,28(35):5153-5159.
17.Shirvani SM,Jiang J,Chang JY,et al.Comparative effectiveness of 5 treatment strategies for earlystage non- small cell lung cancer in the elderly.Int J Radiat Oncol Biol Phys,2012,84(5):1060-1070.
18.Donington J,Ferguson M,Mazzone P,et al.American College of Chest Physicians and Society of Thoracic Surgeons concensus statement for evaluation and management for high-risk patients with stageⅠ non-small cell lung cancer.Chest,2012,142(6):1620-1635.
19.Strauss GM,Herndon JE2nd,Maddaus MA,et al.Adjuvant paclitaxel plus carboplatin compared with observation in stage Ⅰ B non-small-cell lung cancer:CALGB 9633 with the Cancer and Leukemia Group B,Radiation Therapy Oncology Group,and North Central Cancer Treatment Group Study Groups.J Clin Oncol,2008,26(31):5043-5051.
20.Arriagada R,Bergman B,Dunant,et al.Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer.N Engl J Med,2004,350(4):351-360.
21.Pignon JP,Tribodet H,Scagliotti GV,et al.Lung adjuvant cisplatin evaluation:a pooled analysis by the LACE Collaborative Group.J Clin Oncol,2008,26(21):3552-3559.
22.Aupérin A,Le P échoux C,Rolland E,et al.Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer.J Clin Oncol,2010,28(13):2181-2190.
23.O’Rourke N,Roqué I Figuls M,Farré Bernadó N,et al.Concurrent chemoradiotherapy in nonsmall cell lung cancer.Cochrane Database Syst Rev,2010,16(6):CD002140.
24.Curran WJ Jr,Paulus R,Langer CJ,et al.Sequential vs.concurrent chemoradiation for stage Ⅲ nonsmall cell lung cancer:randomized phase Ⅲ trial RTOG 9410.J Natl Cancer Inst,2011,103(19):1452-1460.
25.Albain KS,Crowley JJ,Turrisi AT Ⅲ,et al.Concurrent cisplatin,etoposide,and chest radiotherapy in pathologic stage Ⅲ B non-small-cell lung cancer:A Southwest Oncology Group Phase Ⅰ Study,SWOG 9019.J Clin Oncol,2002,20(16):3454-3360.
26.Kreuter M,Vansteenkiste J,Fischer JR,et al.Randomized phase 2 trial on refinement of early-stage NSCLC adjuvant chemotherapy with cisplatin and pemetrexed versus cisplatin and vinorelbine:the TREAT study.Ann Oncol,2013,24:986-992.
可手术ⅢA期原发性非小细胞肺癌的治疗:
1.Arriagada R,Bergman B,Dunant A,et al.Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med,2004,350(4):351-360.
2.Winton T,Livingston R,Johnson D,et al.Vinorelbine plus cisplatin vs.observation in resected nonsmall-cell lung cancer. N Engl J Med,2005,352(25):2589-2597.
3.Douillard JY,Rosell R,De Lena M,et al.Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage ⅠB-ⅢA non-small-cell lung cancer(Adjuvant Navelbine International Trialist Association [ANITA]):a randomised controlled trial. Lancet Oncol,2006,7(9):719-727.
4.Furuse K,Fukuoka M,Kawahara M,et al.Phase Ⅲ study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin,vindesine,and cisplatin in unresectable stage Ⅲ non-small-cell lung cancer.J Clin Oncol,1999,17(9):2692-2699.
5.Curran WJ,Jr.,Paulus R,Langer CJ,et al.Sequential vs.concurrent chemoradiation for stage Ⅲ nonsmall cell lung cancer:randomized phase Ⅲ trial RTOG 9410.J Natl Cancer Inst,2011,103(19):1452-1460.
6.Fournel P,Robinet G,Thomas P,et al.Randomized phase Ⅲ trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer:Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study.J Clin Oncol,2005,23(25):5910-5917.
7.Zatloukal P,Petruzelka L,Zemanova M,et al.Concurrent versus sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer:a randomized study.Lung Cancer,2004,46(1):87-98.
8.Huber RM,Flentje M,Schmidt M,et al.Simultaneous chemoradiotherapy compared with radiotherapy alone after induction chemotherapy in inoperable stage Ⅲ A or Ⅲ B non-small-cell lung cancer:study CTRT99/97 by the Bronchial Carcinoma Therapy Group.J Clin Oncol,2006,24(27):4397-4404.
9.Clamon G,Herndon J,Cooper R,et al.Radiosensitization with carboplatin for patients with unresectable stage Ⅲ non-small-cell lung cancer:a phase Ⅲ trial of the Cancer and Leukemia Group B and the Eastern Cooperative Oncology Group.J Clin Oncol,1999,17(1):4-11.
10.Belderbos J,Uitterhoeve L,van Zandwijk N,et al.Randomised trial of sequential versus concurrent chemo-radiotherapy in patients with inoperable non-small cell lung cancer(EORTC 08972-22973).Eur J Cancer,2007,43(1):114-121.
11.O'Rourke N,Roque IFM,Farre Bernado N,et al.Concurrent chemoradiotherapy in non-small cell lung cancer.Cochrane Database Syst Rev,2010,16(6):CD002140.
12.Auperin A,Le Pechoux C,Rolland E,et al.Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer.J Clin Oncol,2010,28(13):2181-2190.
13.van Meerbeeck JP,Kramer GW,Van Schil PE,et al.Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage ⅢA-N2 non-small-cell lung cancer. J Natl Cancer Inst,2007,99(6):442-450.
14.Albain KS,Swann RS,Rusch VW,et al.Radiotherapy plus chemotherapy with or without surgical resection for stage Ⅲ non-small-cell lung cancer:a phase Ⅲ randomised controlled trial. Lancet,2009,374(9687):379-386.
15.Pless M,Stupp R,Ris H B,et al.Induction chemoradiation in stage Ⅲ A/N2 non-small-cell lung cancer:a phase 3 randomised trial.Lancet,2015,386(9998):1049-1056.
16.Eberhardt WE,P ttgen C,Gauler TC,et al.Phase Ⅲ Study of Surgery Versus Definitive Concurrent Chemoradiotherapy Boost in Patients With Resectable Stage Ⅲ A(N2)and Selected Ⅲ B Non-Small-Cell Lung Cancer After Induction Chemotherapy and Concurrent Chemoradiotherapy(ESPATUE).J Clin Oncol,2015,33(35):4194-4201.
17.Thomas M,R übe C,Hoffknecht P,et al.Effect of preoperative chemoradiation in addition to preoperative chemotherapy:a randomised trial in stage Ⅲ non-small-cell lung cancer. Lancet Oncol,2008,9(7):636-648.
18.Kwong KF,Edelman MJ,Suntharalingam M,et al.High-dose radiotherapy in trimodality treatment of Pancoast tumors results in high pathologic complete response rates and excellent long-term survival. J Thorac Cardiovasc Surg,2005,129(6):1250-1257.
19.Rusch VW,Giroux DJ,Kraut MJ,et al.Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas:long-term results of Southwest Oncology Group Trial 9416(Intergroup Trial 0160). J Clin Oncol,2007,25(3):313-318.
20.Rusch VW,Giroux DJ,Kraut MJ,et al.Induction chemoradiation and surgical resection for nonsmall cell lung carcinomas of the superior sulcus:Initial results of Southwest Oncology Group Trial 9416(Intergroup Trial 0160). J Thorac Cardiovasc Surg,2001,121(3):472-483.
21.Adebonojo SA,Moritz DM,Danby CA.The results of modern surgical therapy for multiple primary lung cancers. Chest,1997,112(3):693-701.
22.Nakata M,Sawada S,Yamashita M,et al.Surgical treatments for multiple primary adenocarcinoma of the lung.Ann Thorac Surg,2004,78(4):1194-1199.
23.Corso CD,Rutter CE,Wilson LD,et al.Re-evaluation of the role of postoperative radiotherapy and the impact of radiation dose for non-small-cell lung cancer using the National Cancer Database.J Thorac Oncol,2015,10(1):148-155.
24.Urban D,Bar J,Solomon B,et al.Lymph node ratio may predict the benefit of postoperative radiotherapy in non-small-cell lung cancer.J Thorac Oncol,2013,8(7):940-946.
25.Mikell JL,Gillespie TW,Hall WA,et al.Postoperative radiotherapy is associated with better survival in non-small cell lung cancer with involved N2 lymph nodes:results of an analysis of the National Cancer Data Base.J Thorac Oncol,2015,10(3):462-471.
26.Robinson CG,Patel AP,Bradley JD,et al.Postoperative radiotherapy for pathologic N2 non-smallcell lung cancer treated with adjuvant chemotherapy:a review of the National Cancer Data Base.J Clin Oncol,2015,33(8):870-876.
27.Wisnivesky JP,Halm EA,Bonomi M,et al.Postoperative radiotherapy for elderly patients with stageⅢ lung cancer.Cancer,2012,118(18):4478-4485.
28.Perry MC,Kohman LJ,Bonner JA,et al.A phase Ⅲ study of surgical resection and paclitaxel/carboplatin chemotherapy with or without adjuvant radiation therapy for resected stage Ⅲ non-small-cell lung cancer:Cancer and Leukemia Group B 9734.Clin Lung Cancer,2007,8(4):268-272.
29.Le Pé choux C,Dunant A,Pignon JP,et al.Need for a new trial to evaluate adjuvant postoperative radiotherapy in non-small-cell lung cancer patients with N2 mediastinal involvement.J Clin Oncol,2007,25(7):e10-11.
30.Zhong WZ,Wang Q,Mao WM,et al.Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage Ⅱ - Ⅲ A(N1-N2)EGFR-mutant NSCLC(ADJUVANT/CTONG1104):a randomised,open-label,phase 3 study. Lancet Oncol,2018;19(1):139-148.
31.Yue D,Xu S,Wang Q,et al.Efficacy and Safety of Erlotinib vs Vinorelbine/Cisplatin as Adjuvant Therapy for Stage Ⅲ A EGFR Mutant NSCLC Patients.WCLC 2017,OA 16.04.
32.Hanna N,Neubauer M,Yiannoutsos C,et al.Phase Ⅲ study of cisplatin,etoposide,and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage Ⅲ non-small-cell lung cancer:the Hoosier Oncology Group and U.S.Oncology.J Clin Oncol,2008,26(35):5755-5760.
33.Gandara DR,Chansky K,Albain KS,et al.Long-term survival with concurrent chemoradiation therapy followed by consolidation docetaxel in stage Ⅲ B non-small-cell lung cancer:a phase Ⅱ Southwest Oncology Group Study(S9504).Clin Lung Cancer,2006,8(2):116-121.
34.Huber RM,Engel-Riedel W,Kollmeier J,et al.GILT study:Oral vinorelbine(NVBo)and cisplatin(P)with concomitant radiotherapy(RT)followed by either consolidation(C)with NVBo plus P plus best supportive care(BSC)or BSC alone in stage(st)Ⅲ non-small cell lung cancer(NSCLC):Final results of a phase(ph)Ⅲ study.J Clin Oncol,2012;30(15s):7001.
35.Park K,Ahn Y,Ahn J,Ahn M,Kim J,Cho E,et al.A multinational phase Ⅲ randomized trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage Ⅲ non-small cell lung cancer(CCheIN).J Clin Oncol,2014,32(15s):7500.
36.Albain KS,Crowley JJ,Turrisi AT Ⅲ,et al.Concurrent cisplatin,etoposide,and chest radiotherapy in pathologic stage Ⅲ B non-small-cell lung cancer:A Southwest Oncology Group Phase Ⅱ Study,SWOG 9019.J Clin Oncol,2002,20(16):3454-3460.
37.Belani CP,Choy H,Bonomi P,et al.Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer:a randomized phase Ⅱ locally advanced multi-modality protocol.J Clin Oncol,2005,23(25):5883-5891.
38.Senan S,Brade A,Wang LH,et al.PROCLAIM:randomized phase Ⅲ trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J Clin Oncol,2016,34(9):953-962.
39.Wang L,Wu S,Ou G,et al.Randomized phase Ⅱ study of concurrent cisplatin/etoposide or paclitaxel/carboplatin and thoracic radiotherapy in patients with stage Ⅲ non-small cell lung cancer. Lung Cancer,2012,77(1):89-96.
40.Liang J,Bi N,Wu S,et al.Etoposide and Cisplatin vs Paclitaxel and Carboplatin With Concurrent Thoracic Radiotherapy in Unresectable Stage Ⅲ Non-Small Cell Lung Cancer:A Multicenter Randomized Phase Ⅲ Trial.Ann Oncol,2017,28(4):777-783.
41.Bradley JD,Paulus R,Komaki R,et al.Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stageⅢ A orⅢ B non-small-cell lung cancer(RTOG 0617):a randomised,two-by-two factorial phase 3 study. Lancet Oncol,2015,16(2):187-199.
42.Antonia SJ,Villegas A,Daniel D,et al.Durvalumab after chemoradiotherapy in stage Ⅲ Non-Small-Cell lung cancer.N Engl J Med,2017,377(20):1919-1929.
43.Goss GD,O’Callaghan C,Lorimer I,et al.Gefitinib versus placebo in completely resected non-smallcell lung cancer:results of the NCIC CTG BR19 study.J Clin Oncol,2013,31(27):3320-3326.
44.Kelly K,Altorki NK,Eberhardt WE,et al.Adjuvant Erlotinib versus placebo in patients with stage IB-Ⅲ A non-small-cell lung cancer(RADIANT):a randomized,double-blind,phase III trial.J Clin Oncol,2015,33(34):4007-4014.
不可手术ⅢA、ⅢB期原发性非小细胞肺癌的治疗:
1.Curran WJ,Jr,Paulus R,Langer CJ,et al.Sequential vs.concurrent chemoradiation for stage Ⅲ nonsmall cell lung cancer:randomized phase Ⅲ trial RTOG 9410.J Natl Cancer Inst,2011,103(19):1452-1460.
2.Aup érin A,Le P échoux C,Rolland E,et al.Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer.J Clin Oncol,2010,28(13):2181-2190.
3.Chen AB,Neville BA,Sher DJ,et al.Survival outcomes after radiation therapy for stage Ⅲ non-smallcell lung cancer after adoption of computed tomography-based simulation.J Clin Oncol,2011,29(17):2305-2311.
4.Bradley JD,Paulus R,Komaki R,et al.Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stageⅢ A or Ⅲ B non-small-cell lung cancer(RTOG 0617):a randomised,two-by-two factorial phase 3 study.Lancet Oncol,2015,16(2):187-199.
5.Mauguen A,Le Péchoux C,Saunders MI,et al.Hyperfractionated or accelerated radiotherapy in lung cancer:an individual patient data meta-analysis.J Clin Oncol,2012,30(22):2788-2797.
6.Schaake-Koning C,van den Bogaert W,Dalesio O,et al.Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer.N Engl J Med,1992,326(8):524-530.
7.Reymen B,van Baardwijk A,Wanders R,et al.Long-term survival of stage T4N0-1 and single station ⅢA-N2 NSCLC patients treated with definitive chemoradiotherapy using individualised isotoxic accelerated radiotherapy(INDAR).Radiother Oncol,2014,110(3):482-487.
8.Belderbos JS,Kepka L,Spring Kong FM,et al.Report from the International Atomic Energy Agency(IAEA)consultants’ meeting on elective nodal irradiation in lung cancer:non-small-cell lung cancer(NSCLC).Int J Radiat Oncol Biol Phys,2008,72(2):335-342.
9.Yuan S,Sun X,Li M,et al.A randomized study of involved-field irradiation versus elective nodal irradiation in combination with concurrent chemotherapy for inoperable stage Ⅲ nonsmall cell lung cancer.Am J Clin Oncol,2007,30(3):239-244.
10.Chen M,Bao Y,Ma HL,et al.Involved-field radiotherapy versus elective nodal irradiation in combination with concurrent chemotherapy for locally advanced non-small cell lung cancer:a prospective randomized study.Biomed Res Int,2013,2013:371819.
11.Wang L,Wu S,Ou G,et al.Randomized phase Ⅱ study of concurrent cisplatin/ etoposide or paclitaxel/carboplatin and thoracic radiotherapy in patients with stage Ⅲ non-small cell lung cancer.Lung Cancer,2012,77(1):89-96.
12.Garrido P,Rosell R,Arellano A,et al.Randomized phase Ⅱ trial of non-platinum induction or consolidation chemotherapy plus concomitant chemoradiation in stage Ⅲ NSCLC patients:mature results of the Spanish Lung Cancer Group 0008 study.Lung Cancer,2013,81(1):84-90.
13.Belderbos J,Uitterhoeve L,van Zandwijk N,et al.Randomised trial of sequential versus concurrent chemo-radiotherapy in patients with inoperable non-small cell lung cancer(EORTC 08972-22973).Eur J Cancer,2007,43(1):114-121.
14.Ahn JS,Ahn YC,Kim JH,et al.Multinational randomized phase Ⅲ trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage Ⅲ nonsmall-cell lung cancer:KCSG-LU05-04.J Clin Oncol,2015,33(24):2660-2666.
15.Wu YL,Lu S,Cheng Y,Zhou C,et al.Efficacy and safety of pemetrexed/ cisplatin versus gemcitabine/cisplatin as first-line treatment in Chinese patients with advanced nonsquamous non-small cell lung cancer.Lung Cancer,2014,85(3):401-407.
16.Choy H,Gerber DE,Bradley JD,et al.Concurrent pemetrexed and radiation therapy in the treatment of patients with inoperable stage Ⅲ non-small cell lung cancer:a systematic review of completed and ongoing studies.Lung Cancer,2015,87(3):232-240.
17.Vokes EE,Herndon JE,II,Kelley MJ,et al.Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage Ⅲ non-small-cell lung cancer:Cancer and Leukemia Group B.J Clin Oncol,2007,25(13):1698-1704.
18.van Meerbeeck JP,Kramer GW,Van Schil PE,et al.Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage ⅢA-N2 non-small-cell lung cancer.J Natl Cancer Inst,2007,99(6):442-450.
19.Hanna N,Neubauer M,Yiannoutsos C,et al.PhaseⅢ study of cisplatin etoposide,and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage Ⅲ non-small-cell lung cancer:the Hoosier Oncology Group and U.S.Oncology.J Clin Oncol,2008,26(35):5755-5760.
20.Vansteenkiste J,De Ruysscher D,Eberhardt WE,et al.Early and locally advanced non-small-cell lung cancer(NSCLC):ESMO Clinical Practice Guidelines for diagnosis,treatment and follow-up.Ann Oncol,2013,24 Suppl 6:vi89-98.
21.Eberhardt W,Pttgen C,Gauler TC,et al.Phase Ⅲ Study of Surgery Versus Definitive Concurrent Chemoradiotherapy Boost in Patients With Resectable Stage Ⅲ A(N2)and Selected Ⅲ B Non-Small-Cell Lung Cancer After Induction Chemotherapy and Concurrent Chemoradiotherapy(ESPATUE).J Clin Oncol,2015;33(35):4194-201.
22.Antonia SJ,Villegas A,Daniel D,et al.Durvalumab after chemoradiotherapy in stage III Non-Small-Cell lung cancer.N Engl J Med,2017,377(20):1919-1929.
Ⅳ期驱动基因阳性非小细胞肺癌的治疗:
1.Mok TS,Wu YL,Thongprasert S,et al.Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma.N Engl J Med,2009,361(10):947-957.
2.Han JY,Park K,Kim SW,et al.First-SIGNAL:first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung.J Clin Oncol,2012,30(10):1122-1128.
3.Maemondo M,Inoue A,Kobayashi K,et al.Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR.N Engl J Med,2010,362(25):2380-2388.
4.Mitsudomi T,Morita S,Yatabe Y,et al.Gefitinib versus cisplatin plus docetaxel in patients with nonsmall-cell lung cancer harbouring mutations of the epidermal growth factor receptor(WJTOG3405):an open label,randomised phase 3 trial.Lancet Oncol,2010,11(2):121-128.
5.Rosell R,Carcereny E,Gervais R,et al.Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer(EURTAC):a multicentre,open-label,randomised phase 3 trial.Lancet Oncol,2012,13(3):239-246.
6.Zhou C,Wu YL,Chen G,et al.Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer(OPTIMAL,CTONG-0802):a multicentre,open-label,randomised,phase 3 study.Lancet Oncol,2011,12(8):735-742.
7.Wu YL,Zhou C,Liam CK,et al.First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer:analyses from the phase Ⅲ,randomized,open-label,ENSURE study.Ann Oncol,2015,26(9):1883-1889.
8.Sequist LV,Yang JCH,Yamamoto N,et al.Phase Ⅲ study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutation.J Clin Oncol,2013,31(27):3327-3334.
9.Wu YL,Zhou C,Hu CP,et al.Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations(LUX-Lung 6):an open-label,randomised phase 3 trial.Lancet Oncol,2014,15(2):213-222.
10.Shi YK,Wang L1,Han BH,Li W,et al.First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma(CONVINCE):a phase 3,open-label,randomized study.Ann Oncol.2017,28(10):2443-2450.
11.Park K,Tan EH,O'Byrne K,et al.Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer(LUX-Lung 7):a phase 2B,open-label,randomised controlled trial.Lancet Oncol,2016,17(5):577-589.
12.Yang JC,Wu YL,Schuler M,et al.Afatinib versus cisplatin-based chemotherapy for EGFR mutationpositive lung adenocarcinoma(LUX-Lung 3 and LUX-Lung 6):analysis of overall survival data from two randomised,phase 3 trials.Lancet Oncol,2015,16(2):141-151.
13.Xu CR,Wu YL,Hu CP,et al.Afatinib vs cisplatin/gemcitabine for the first-line treatment of Chinese patients with advanced EGFR-mutation positive(EGFRm+)NSCLC:subgroup analysis of the LUXLung 6 trial.2017 CSCO Poster B0858.
14.Yang JCH,Sequist LV,Geater SL,et al.Clinical activity of afatinib in patients with advanced nonsmall-cell lung cancer harbouring uncommon EGFR mutations:a combined post-hoc analysis of LUXLung 2,LUX-Lung 3,and LUX-Lung 6.Lancet Oncol,2015,16(7):830-838.
15.Wu YL,Cheng Y,Zhou X,et al.Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer(ARCHER 1050):a randomised,open-label,phase 3 trial.Lancet Oncol.2017;18(11):1454-1466.
16.Zhou Q,Hu C,Li W,et al.Dacomitinib vs Gefitinib for First-Line(1L)T reatment of Advanced EGFR+Non-Small-Cell Lung Cancer(NSCLC)in Chinese Patients(ARCHER 1050).2017 CSCO plenary Session Oral.
17.Soria JC,Ohe Y,Vansteenkiste J,et al.Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer.N Engl J Med.2018;378(2):113-125.
18.Wu YL,Lee JS,Thongprasert S,et al.Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer(FASTACT-2):a randomised,double-blind trial.Lancet Oncol,2013,14(8):777-786.
19.Cheng Y,Murakami H,Yang PC,et al.Randomized Phase II Trial of Gefitinib With and Without Pemetrexed as First-Line Therapy in Patients With Advanced Nonsquamous Non-Small-Cell Lung Cancer With Activating Epidermal Growth Factor Receptor Mutations.J Clin Oncol,2016,34(27):3258-3266.
20.Seto T,Kato T,Nishio M,et al.Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations(JO25567):an open-label,randomised,multicentre,phase 2 study.Lancet Oncol 2014;15:1236-1244.
21.S.Novello,F.Barlesi,R.Califano,et al.Metastatic Non-Small-Cell Lung Cancer:ESMO Clinical Practice Guidelines.Ann Oncol(2016)27(suppl 5):v1-v27.
22.日本肺癌学会临床实践指南2017版(http://www.haigan.gr.jp/modules/guideline/index.php?content_id=3).
23.Yang JJ,Zhou C,Huang Y,et al.Icotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases(BRAIN):a multicentre,phase 3,open-label,parallel,randomised controlled trial.Lancet Respiratory Med 2017;5(9):707-716.
24.Vansteenkiste J,Ramalingam SS,T Reungwetwattana,et al.CNS response to Osimertinib vs standardof-care EGFR-TKI as first-line treatment in patients with EGFRm advanced NSCLC:FLAURA.ESMO Asia 2017 LBA5.
25.Yang JJ,Chen HJ,Yan HH,et al.Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer.Lung Cancer,2013,79(1):33-39.
26.Weickhardt AJ,Scheier B,Burke JM,et al.Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer.J Thorac Oncol,2012,7(12):1807-1814.
27.Conforti F,Catania C,Toffalorio F,et al.EGFR tyrosine kinase inhibitors beyond focal progression obtain a prolonged disease control in patients with advanced adenocarcinoma of the lung.Lung Cancer,2013,81(3):440-444.
28.Shukuya T,Takahashi T,Naito T,et al.Continuous EGFR-TKI administration following radiotherapy for non-small cell lung cancer patients with isolated CNS failure.Lung Cancer,2011,74(3):457-461.
29.Yu HA,Sima CS,Huang J,et al.Local therapy with continued EGFR tyrosine kinase inhibitor therapy as a treatment strategy in EGFR-mutant advanced lung cancers that have developed acquired resistance to EGFR tyrosine kinase inhibitors.J Thorac Oncol,2013,8(3):346-351.
30.Hong SH,Jeon E,Kim YS,et al.Clinical outcomes of continuing EGFR receptor tyrosine kinase inhibitors after recist progression of bone metastasis in EGFR-mutant NSCLC.Lung Cancer,2013,80(Suppl 1):S35.
31.Parra HJS,Chiari R,Bearz A,et al.A retrospective analysis of the clinical responses to EGFR-tyrosine kinase inhibitor(EGFR-TKI)continuous treatment beyond single site disease progression in metastatic nonsmall cell lung cancer patients who benefited from prior egfrtki therapy.J Thorac Oncol,2011,6(6 Suppl 2):S1254.
32.Park K,Yu CJ,Kim SW,et al.First-line erlotinib therapy until and beyond response evaluation criteria in solid tumors progression in asian patients with epidermal growth factor receptor mutation-positive nonsmall-cell lung cancer:the ASPIRATION study.JAMA Oncol,2016,2(3):305-312.
33.Hosomi Y,Tanai C,Yoh K,et al.Observational study of treatment with epidermal growth factor receptor tyrosine kinase inhibitors(EGFR-TKI)in activating EGFR-mutation-positive(EGFRm+)advanced or recurrent non-small cell lung cancer(NSCLC)after radiologic progression to first-line therapy with EGFR-TKI.J Clin Oncol,2014,32(15s suppl):abstr 8071.
34.Chen Q,Quan Q,Ding L,et al.Continuation of epidermal growth factor receptor tyrosine kinase inhibitor treatment prolongs disease control in non-small-cell lung cancers with acquired resistance to EGFR tyrosine kinase inhibitors.Oncotarget,2015,6(28):24904-24911.
35.Soria JC,Wu YL,Nakagawa K,et al.Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib(IMPRESS):a phase 3 randomised trial.Lancet Oncol,2015,16(