 去看看
去看看


1 胃癌诊断
1.1 诊断基本原则
胃癌治疗前基本诊断手段主要包括内镜和影像学检查,用于胃癌的定性诊断、定位诊断和分期诊断。其他还包括体格检查、实验室检查、内镜(超声内镜和细针穿刺)、转移灶活检以及诊断性腹腔镜探查和腹腔灌洗液评价。内镜活检组织病理学诊断是胃癌确诊和治疗的依据。胸腹盆部CT检查是治疗前分期的基本手段,MRI、腹腔镜探查及PET分别作为CT疑诊肝转移、腹膜转移及全身转移时的备选手段。影像学报告应提供涉及cTNM分期的征象描述,并给出分期意见。胃癌术后系统组织病理学诊断(pTNM分期)为明确胃癌的组织学类型、全面评估胃癌病期进展和判断患者预后、制定有针对性的个体化治疗方案提供必要的组织病理学依据。目前以HER2表达状态为依据的胃癌分子分型是其分子靶向治疗的依据,所有经病理诊断证实为胃腺癌的病例均有必要进行HER2检测。
1.2 影像内镜
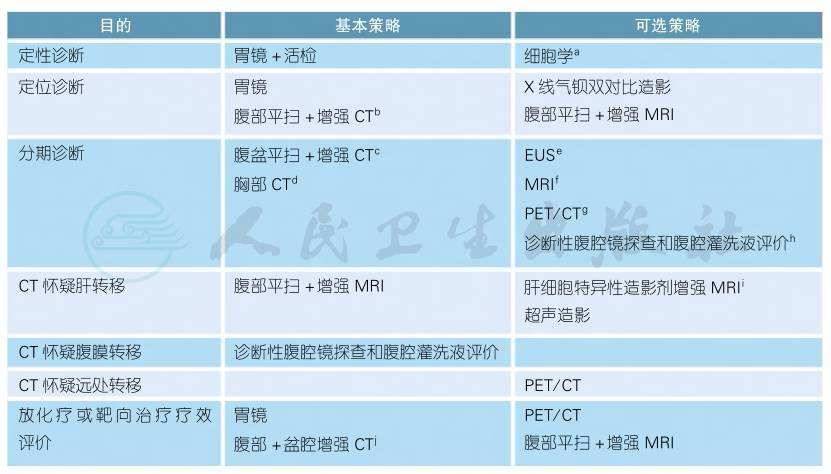
注释
a.胃镜反复活检无法确定病理诊断时,腹水/胸腔积液细胞学或转移灶的病理学检测可作为定性诊断依据。
b.确认胃镜定位,辅助手术术式制定;应报告胃癌的位置(食管胃交界区、胃底、胃体、胃窦、幽门、大弯、小弯,前壁、后壁)及上下界累及范围。
c.一项meta分析汇集40个临床研究,共计3758例胃癌患者,CT对T分期准确率75%~85%,N分期准确率66%,M分期准确率82%[1]。应通过低张、气/水充盈等手段保证胃腔的充分充盈扩张[2,3],多期增强扫描[2],需结合多平面重组图像进行诊断[2-4],结合多平面重组技术(轴位、冠状及矢状位)可提高T分期准确率10%~20%[4]。不建议腹部平扫CT检查,不利于胃癌原发灶分期和淋巴结的检出,如有CT增强扫描禁忌,建议MRI或EUS。
d.胸部CT可较X线平片更好地检出和显示肺部转移灶[3]。临床应用中应根据情况选择平扫或增强。食管胃交界部癌需要判断范围及纵隔淋巴结转移情况时应行胸部增强扫描。
e.推荐有条件的中心开展。一项关于EUS胃癌分期的meta分析指出,EUS胃癌T分期准确率75%[5]。AJCC/UICC第8版分期中EUS为cT分期的推荐手段[2]。
f.虽然小样本研究发现MR成像的软组织分辨率优于CT,但应用于临床T分期的影像学评估仍存在较多局限性[2,3,6]。目前可作为CT增强禁忌患者的替代检查方法。
g.可辅助胃癌分期[7],但不作常规??
1.2 影像内镜
1.Seevaratnam R, Cardoso R, Mcgregor C, et al. How useful is preoperative imaging for tumor, node,metastasis(TNM) staging of gastric cancer? A meta-analysis. Gastric Cancer, 2012, 15 Suppl 1(1):S3-18.
2.Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual.8th ed. New York:Springer,2017.
3.Allum WH Blazeby JM,Griffin SM,et al. Guidelines for the management of oesophageal and gastric cancer. Gut, 2011, 60(11): 1449-1472.
4.Chen C, Hsu JD, Kang W, et al. Gastric cancer: preoperative local staging with 3D multi-detector row CT-correlation with surgical and histopathologic results. Radiology, 2007, 242(2): 472-482.
5.Cardoso R,Coburn N,Seevaratnam R,et al. A systematic review and meta-analysis of the utility of EUS for preoperative staging for gastric cancer. Gastric Cancer, 2012, 15 Suppl 1(1): S19-26.
6.Giganti F, Orsenigo E, Arcidiacono P G, et al. Preoperative locoregional staging of gastric cancer: is there a place for magnetic resonance imaging?Prospective comparison with EUS and multidetector computed tomography. Gastric Cancer, 2016, 19(1): 1-10.
7.Chen J, Cheong JH, Yun MJ, et al. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer, 2005, 103(11): 2383-2390.
8.Mezhir JJ,Shah MA,Jacks LM, et al. Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Ann Surg Oncol, 2010, 17(12): 3173-3180.
9.Kim YK,Lee MW,Lee WJ, et al. Diagnostic accuracy and sensitivity of diffusion weighted and of gadoxetic acid enhanced 3-T MR imaging alone orin combination in the detection of small liver metastasis(≤ 1.5 cm in diameter).Invest Radiol, 2012, 47(3): 159-166.
10.Yoshikawa T, Tanabe K, Nishikawa K, et al. Accuracy of CT staging of locally advanced gastric cancer after neoadjuvant chemotherapy:cohort evaluation within a randomized phaseⅡ study. Ann Surg Oncol, 2014, 21 Suppl 3(3): S385-389.
1.3.1 组织病理学诊断
1.Xu G,Zhang W,Lv Y,et al. Risk factors for under-diagnosis of gastric intraepithelial neoplasia and early gastric carcinoma in endoscopic forceps biopsy in comparison with endoscopic submucosal dissection in Chinese patients. Surg Endosc, 2016, 30(7): 2716-2722.
2.Zhou PH,Schumacher B, Yao LQ,et al. Conventional vs.water jet-assisted endoscopic submucosal dissection in early gastric cancer: a randomized controlled trial. Endoscopy, 2014, 46(10):836-843.
3.中华人民共和国卫生计划生育委员会.《胃癌规范化诊疗指南 (试行)》2013版.
4.梁丽,张继新,戎龙,等.80例早期胃癌及其癌前病变内镜黏膜下剥离术标本的处理及病理学评估.中华消化内镜杂志,2016,33(9):589-597.
5.AJCC Cancer Staging Manual.8th edition. New York: Springer, 2016.
6.全国胃癌病理协作组.1477早期胃癌病例分析--(一)早期胃癌的病理分析.(二)特殊大体类型的早期胃癌.中华消化杂志,1990,10(5):287-290,10(6):341-343.
7.Lauren B. The two histological main types of gastric carcinoma: diffuse and so-called intestinal•type carcinoma. An attempt at a histoclinical classification. Acta Pathol Microbiol Scand, 1965, 64: 31-34.
8.Sun Z, Zhu GL,Lu C, et al. A novel subclassification of pT2 gastric cancers according to the depth of muscularis propria invasion:superficial muscularis propria versus deep muscularis propria/subserosa.Ann Surg, 2009, 249(5): 768-775.
9.NCCN Practice Guidelines in Oncology(Gastric cancer)-v.3.2016(www.nccn.org).
10.薛卫成,樊祥山,孟刚整理.胃癌相关标志物免疫组化指标选择专家共识 (2014).临床与试验病理学杂志,2014,30(9):951-953.
1.3.2 分子分型诊断
1.中国临床肿瘤学会抗肿瘤药物安全管理专家委员会、中国抗癌协会胃癌专业委员会、肿瘤病理专业委员会.HER阳性晚期胃癌分子靶向治疗的中国专家共识 (2016版).临床肿瘤学杂志,2016, 21(9): 831-839.
2.Sheng WQ, Huang D, Ying JM, et al. HER2 status in gastric cancers: a retrospective analysis from four Chinese representative clinical centers and assessment of its prognostic significance. Ann 0ncol,2013, 24(9): 2360-2364.
3.Huang D, Lu N, Fan Q, et al. HER2 status in gastric and gastroesophageal junction cancer assessed by local and central laboratories: Chinese results of the HER2-EAGLE study. Plos One, 2013, 8(11):e80290.
4.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric and gastro-oesophageal junction cancer(ToGA): a phase 3, open-label, randomized controlled trial. Lancet, 2010, 376(9742):687-697.
5.Qiu MZ, Li Q, Wang ZQ, et al. HER2-positive patients receiving trastuzumab treatment have a comparable prognosis with HER2-negative advanced gastric cancer patients:a prospective cohort observation.Int J Cancer, 2014, 134(10): 2468-2477.
6.Gong J, Liu T, Fan Q, et al. Optimal regimen of trastuzumab in combination with oxaliplatin/capecitabine in first-line treatment of HER2-positive advanced gastric cancer(CGOG1001): a multicenter,phaseⅡ trial. BMC Cancer,2016,16:68.
7.Ryu MH,Yoo C,Kim JG,et al. Multicenter phaseⅡ study of trastuzumab in combination with capecitabine and oxaliplatin for advanced gastric cancer. Eur J cancer, 2015, 5l(4): 482-488.
8.Kim KM,Bilous M,Chu KM,et al. Human epidermal growth factor receptor 2 testing in gastric cancer: recommendations of an Asia-Pacmc taskforce. Asia Pac J clin Oncol, 2014, 10(4): 297-307.
9.Wang T, Hsieh ET, Henry P, et al. Matched biopsy and resection specimens of gastric and gastroesophageal adenocarcinoma show high concordance in HER2 status. Hum Pathol, 2014, 45(5):970-975.
10.《胃癌HER2检测指南 (2016版)》专家组.胃癌HER2检测指南 (2016版).中华病理学杂志,2016,45(8):528-532.
2.1.1.1 早期胃癌的治疗
1.中华人民共和国卫生部.《胃癌诊疗规范 (2011年版)》.
2.Kim JJ, Lee JH, Jung HY, et al. EMR for early gastric cancer in Korea: a multicenter retrospective study. Gastrointestinal endoscopy, 2007, 66(4): 693-700.
3.Isomoto H, Shikuwa S, Yamaguchi N, et al. Endoscopic submucosal dissection for early gastric cancer:a large-scale feasibility study. Gut, 2009, 58: 331-336.
4.Nakamoto S, Sakai Y, Kasanuki J, et al. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy,2009, 41: 746-750.
5.Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. Journal of gastroenterology, 2006, 41(10): 929-942.
6.Japanese gastric cancer treatment guidelines 2014(ver.4).Gastric cancer:official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association.2016.
7.Park YM,Cho E,Kang HY,et al. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer:a systematic review and meta-analysis. Surg Endosc, 2011, 25: 2666-2677.
8.Ono H,Yao K,Fujishiro M,et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Digestive endoscopy:official journal of the Japan Gastroenterological Endoscopy Society, 2016, 28(1): 3-15.
9.TakizawaK,Kawata N,Tanaka M,et al. Treatment for intramucosal gastric cancer with mixed type histology(differentiated and undifferentiated).Stomach Intest, 2013, 48: 1567-1579.
10.Ahn JY, Jung HY, Choi KD. Endoscopic and oncologic outcomes after endoscopic resection for early gastric cancer: 1370 cases of absolute and extended indications. Gastrointest Endosc, 2011, 74: 485-493.
11.Nakamura K,Katai H,Mizusawa J,et al. A phaseⅢ study of aparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric Cancer(JCOG0912).Jpn J Clin Oncol, 2013, 43: 324-327.
12.Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report-a phase Ⅲ multicenter, prospective, randomized Trial(KLASS Trial).Ann Surg, 2010, 251: 417-420.
13.Katai H,Mizusawa J,Katayama H,et al. Short-term surgical outcomes from a phaseⅢstudy of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage ⅠA/ⅠB gastric cancer: Japan Clinical Oncology Group Study JCOG0912.Gastric cancer:official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association.2016.
14.Sakuramoto S, Sasako M, Yamaguchi T, et al; ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med, 2007, 357(18): 1810-1820.
15.Sasako M,Sakuramoto S,Katai H,et al. Five-year outcomes of a randomized phaseⅢ trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage Ⅱ orⅢ gastric cancer. J Clin Oncol,2011, 29(33): 4387-4393.
16.Bang YJ, Kim YW, Yang HK, et al. CLASSIC trial investigators. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy(CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet, 2012, 379(9813): 315-321.
17.Lee J1,Lim DH,Kim S,et al. PhaseⅢ trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol, 2012, 30(3): 268-273.
2.1.1.2.3 围术期治疗
1.中华人民共和国卫生部.《胃癌诊疗规范 (2011年版)》.
2.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014(ver.4).Gastric Cancer, 2017, 20(1): 1-19.
3.Sasako,M. Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol, 2006, 7(8): 644-651.
4.Biondi,A. Does a minimum number of 16 retrieved nodes affect survival in curatively resected gastric cancer?Eur J Surg Oncol, 2015, 41(6): 779-786.
5.Zhang CH,Wu AW,Li ZY,et al. Analysis of splenic hilar lymph node metastasis in advanced gastric cancer and dissection techniques. Zhonghua Wei Chang Wai Ke Za Zhi, 2011, 14(8):589-592.
6.Sasada S, Ninomiya M,Nishizaki M, et al. Frequency of lymph node metastasis to the splenic hilus and effect of splenectomy in proximal gastric cancer. Anticancer Res, 2009, 29(8): 3347-3351.
7.Aoyagi K,Kouhuji K,Miyagi M,et al. Prognosis of metastatic splenic hilum lymph node in patients with gastric cancer after total gastrectomy and splenectomy. World J Hepatol, 2010, 2(2):81-86.
8.Sano T, Sasako M, Mizusawa J, et al. Randomized Controlled Trial to Evaluate Splenectomy in Total Gastrectomy for Proximal Gastric Carcinoma. Ann Surg, 2017, 265(2): 277-283.
9.Liang YX,Liang H,Ding XW,et al. Risk factors for group 14 v lymph node metastasis in advanced gastric cancer. Zhonghua Xiao Hua Wai Ke Za Zhi, 2013, 16(7): 632-636.Chinese.
10.Liang H,Deng J. Evaluation of rational extent lymphadenectomy for local advanced gastric cancer.Chin J Cancer Res, 2016, 28: 397-403.
11.Eom BW,Joo J,Kim YW,et al. Improved survival after adding dissection of the superior mesenteric vein lymph node(14v)to standard D2 gastrectomy for advanced distal gastric cancer. Surgery, 2014,155:408-416.
12.Sasako,M. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med, 2008, 359(5): 453-462.
13.Huscher, C. G.Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg, 2005, 241(2): 232-237.
14.Tanimura, S. Laparoscopic gastrectomy for gastric cancer: experience with more than 600 cases. Surg Endosc, 2008, 22(5): 1161-1164.
15.Kim, H. H.Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol, 2014, 32(7): 627-633.
16.Hu Y,Huang C, Sun Y, et al. Morbidity and Mortality of Laparoscopic Versus Open D2 Distal Gastrectomy for Advanced Gastric Cancer: A Randomized Controlled Trial. J Clin Oncol, 2016, 34(12):1350-1357.
17.Kang KC. Korean Laparoscopic Gastrointestinal Surgery Study(KLASS)Group. Comparison of BillrothⅠand BillrothⅡreconstructions after laparoscopy-assisted distal gastrectomy: a retrospective analysis of large-scale multicenter results from Korea. Surg Endosc, 2011, 25 (6):1953-1961.
18.Shiraishi N. Gastric tube reconstruction prevented esophageal reflux after proximal gastrectomy. Gastric Cancer, 1998, 1(1): 78-79.
19.Souya Nunobe, Billroth 1 versus Roux-en-Y reconstructions: a quality-of-life survey at 5 year. Int J Clin Oncol, 2007, 12(6): 433-439.
20.梁寒.胃癌根治手术写真.天津:天津科技翻译出版有限公司,2013.
21.Fein M. Long-term benefits of Roux-en-Y pouch reconstruction after total gastrectomy:a randomized trial. Ann Surg, 2008, 247(5): 759-765.
22.Wang W, Sun Z, Deng J, et al. Integration and analysis of associated data in surgical treatment of gastric cancer based on multicenter, high volume databases, Zhonghua Wei Chang Wai Ke Za Zhi, 2016,19(2):179-185.
23.Cunningham D, Allum WH, Stenning SP, et al. MAGIC Trial Participants. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med, 2006, 355(1):11-20.
24.Ychou M, Boige V, Pignon JP,et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phaseⅢ trial. J Clin Oncol, 2011, 29(13): 1715-1721.
25.Sumpter K, Harper-Wynne C, Cunningham D, et al. Report of two protocol planned interim analyses in a randomised multicentre phaseⅢstudy comparing capecitabine with fluorouracil and oxaliplatin with cisplatin in patients with advanced oesophagogastric cancer receiving ECF. Br J Cancer, 2005, 92(11):1976-1983.
26.Cunningham D1, Starling N, Rao S, et al. Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med, 2008, 358(1): 36-46.
27.Li ZY, Koh CE,Bu ZD, et al. Neoadjuvant chemotherapy with FOLFOX:improved outcomes in Chinese patients with locally advanced gastric cancer. J Surg Oncol, 2012, 105(8): 793-799.
28.Kochi M,Fujii M,Kanamori N,et al. PhaseⅡ Study of Neoadjuvant Chemotherapy With S-1 and CDDP in Patients With Lymph Node Metastatic StageⅡ orⅢ Gastric Cancer. Am J Clin Oncol, 2017,40(1):17-21.
29.Li T,Chen L. Efficacy and safety of SOX regimen as neoadjuvant chemotherapy for advanced gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi, 2011, 14(2): 104-106.
30.Stahl M,Walz M K,Stuschke M,et al. PhaseⅢcomparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction.J Clin Oncol, 2009, 27(6): 851-856.
31.Ajani J A,Winter K,Okawara G S,et al. PhaseⅡ trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma(RTOG 9904):quality of combined modality therapy and pathologic response. J Clin Oncol, 2006, 24(24): 3953-3958.
32.Leong T,Smithers B M,Michael M,et al. TOPGEAR:a randomised phaseⅢ trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer(an international,intergroup trial of the AGITG/TROG/EORTC/NCIC CTG).BMC Cancer, 2015, 15: 532.
33.Sarela AI, Lefkowitz R, Brennan MF, et al. Selection of patients with gastric adenocarcinoma for laparoscopic staging. Am J Surg, 2006, 191(1): 134-138.
34.Bang YJ, Kim YW, Yang HK, et al. CLASSIC trial investigators. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy(CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet, 379(9813): 315-321.
35.Lee J, Lim DH, Kim S, et al. PhaseⅢ trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial J Clin Oncol, 2012, 30(3): 268-273.
36.Sakuramoto S, Sasako M, Yamaguchi T, et al. ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med, 2007, 357(18): 1810-1820.
37.Sasako M,Sakuramoto S,Katai H,et al. Five-year outcomes of a randomized phaseⅢ trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stageⅡorⅢgastric cancer. J Clin Oncol, 2011, 29(33): 4387-4393.
38.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med, 2001, 345(10):72.
39.Stiekema J, Trip AK, Jansen EP, et al. The prognostic significance of an R1 resection in gastric cancer patients treated with adjuvant chemoradiotherapy. Ann Surg Oncol, 2014, 21 (4):1107-1114.
2.1.2 不可手术切除胃癌的综合治疗
1.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med, 2012, 366: 2074-2084.
2.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase Ⅲ trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781.J Clin Oncol, 2008, 26(7): 1086-1092.
3.Khushalani NI, Leichman CG, Proulx G, et al. Oxaliplatin in combination with protracted-infusion fluorouracil and radiation: report of a clinical trial for patients with esophageal cancer. J Clin Oncol, 2002,20(12):2844-2850.
4.Ajani JA,Mansfield PF,Crane CH,et al. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma:degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol, 2005, 23(6): 1237-1244.
5.Ajani JA,Winter K,Okawara GS,et al. PhaseⅡ trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma(RTOG 9904):quality of combined modality therapy and pathologic response. J Clin Oncol, 2006, 24(24): 3953-3958.
6.Hu JB,Sun XN,Gu BX, Wang Q, Hu WX. Effect of intensity modulated radiotherapy combined with s-1-based chemotherapy in locally advanced gastric cancer patients. Oncol Res Treat, 2014, 37(1-2):11-16.
7.Lee J,Lim dH,Kim S,et al. PhaseⅢ trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol, 2012, 30(3): 268-273.
8.Inoue T, Yachida S, Usuki H, et al. Pilot feasibility study of neoadjuvant chemoradiotherapy with S-1 in patients with locally advanced gastric cancer featuring adjacent tissue invasion or JGCA bulky N2 lymph node metastases. Ann Surg Oncol, 2012, 19(9): 2937-2945.
9.Wang X, Zhao DB, Jin J, et al. A Randomized PhaseⅡTrial of Neoadjuvant Chemotherapy Compared with Chemoradiation Therapy in Locally Advanced Gastroesophageal and Gastric Adenocarcinoma:Preliminary Results. J Radiat Oncol Biol Phys, 2016, 96(2): Supplement 32.
10.Ajani JA, Mansfield PF, Janjan N, et al. Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol, 2004, 22(14):2774-2780.
11.Gastrointestinal Tumor Study Group. A comparison of combination chemotherapy and combined modality therapy for locally advanced gastric carcinoma. Cancer, 1982, 49: 1771-1777.
12.Gunderson LL, Hoskins RB, Cohen AC, et al. Combined modality treatment of gastric cancer. Int J Radiat Oncol Biol Phys, 1983, 9(7): 965-975.
13.Moertel CG, Childs DS, Reitemeier RJ, et al. Combined 5-fluorouracil and supervoltage radiation therapy of locally unresectable gastrointestinal cancer. Lancet, 1969, 2: 865-867.
14.Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol, 2006, 24: 2903-2909.
15.Al-Batran SE, Hartmann JT, Probst S, et al. PhaseⅢtrial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol, 2008, 26(9): 1435-1442.
16.Mantell BS. Radiotherapy for dysphagia due to gastric carcinoma. Br J Surg, 1982, 69(2): 69-70.
17.Coia LR,Paul AR, Engstrom PF. Combined radiation and chemotherapy as primary management of adenocarcinoma of the esophagus and gastroesophageal junction. Cancer, 1988, 15, 61(4): 643-649.
18.Kim MM,Rana V,Janjan NA,et al. Clinical benefit of palliative radiation therapy in advanced gastric cancer. Acta Oncol, 2008, 47(3): 421-427.
19.Minn AY,Hsu A,La T, et al. Comparison of intensity-modulated radiotherapy and 3-dimensional conformal radiotherapy as adjuvant therapy for gastric cancer. Cancer, 2010, 116(16): 3943-3952.
20.Wang X, Li G, Zhang Y, et al. Single-arc volumetric-modulated arc therapy(sVMAT)as adjuvant treatment for gastric cancer:dosimetric comparisons with three-dimensional conformal radiotherapy(3DCRT)and intensity-modulated radiotherapy(IMRT).Med Dosim, 2013, 38(4): 395-400.
2.2.1 晚期转移性胃癌的药物治疗选择
1.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med, 2008, 358: 36-46.
2.Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomized phase Ⅲ noninferiority trial. Ann Oncol,2009, 20: 666-673.
3.Luo HY,Xu RH,Wang F,et al. PhaseⅡ trial of XELOX as first-line treatment for patients with advanced gastric cancer. Chemotherapy, 2010, 56(2): 94-100.
4.Van Cutsem E,Moiseyenko VM,Tjulandin S,et al. PhaseⅢ study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer:a report of the V325 Study Group. J Clin Oncol, 2006, 24: 4991-4997.
5.Wang J, Xu R, Li J, et al. Randomized multicenter phase Ⅲ study of a modified docetaxel and cisplatin plus fluorouracil regimen compared with cisplatin and fluorouracil as first-line therapy for advanced or locally recurrent gastric cancer. Gastric Cancer, 2016, 19(1): 234-244.
6.Koizumi W,Narahara H,Hara T,et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer(SPIRITS trial): a phaseⅢ trial. Lancet Oncol, 2008, 9: 215-221.
7.Ajani JA,Rodriguez W,Bodoky G,et al. Multicenter phaseⅢ comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastro-esophageal adenocarcinoma study:the FLAGS trial. J Clin Oncol, 2010, 28: 1547-1553.
8.Li YH, Qiu MZ,Xu JM, et al. S-1 plus cisplatin versus fluorouracil plus cisplatin in advanced gastric or gastro-esophageal junction adenocarcinoma patients: a pilot study. Oncotarget, 2015, 6(33): 35107-35115.
9.Qiu MZ, Wei XL, Zhang DS, et al. Efficacy and safety of capecitabine as maintenance treatment after first-line chemotherapy using oxaliplatin and capecitabine in advanced gastric adenocarcinoma patients:a prospective observation. Tumour Biol, 2014, 35(5): 4369-4375.
10.Hawkes E, Okines AF,Papamichael D,et al. Docetaxel and irinotecan as second-line therapy for advanced oesophagogastric cancer. Eur J Cancer, 2011, 47(8): 1146-1151.
11.Hironaka S1, Ueda S, Yasui H, et al. Randomized, open-label, phaseⅢ study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol,2013, 31(35): 4438-4444.
12.Kim KC, Koh YW, Chang HM, et al. Evaluation of HER2 protein expression in gastric carcinomas:comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann Surg Oncol, 2011, 18: 2833-2840.
13.Gomez L, Concha A, Garcia-Caballero T, et al. Assessment of HER2 status from an epidemiology study in tumor tissue samples of gastric and gastro-esophageal junction cancer:Spanish results of the HEREAGLE study. J Clin Oncol, 2012, 30(Suppl): a4089.
14.Sheng WQ, Huang D, Ying JM, et al. HER2 status in gastric cancers: a retrospective analysis from four Chinese representative clinical centers and assessment of its prognostic significance. Ann Oncol,2013, 24(9): 2360-2364.
15.Qiu M, Zhou Y,Zhang X, et al. Lauren classification combined with HER2 status is a better prognostic factor in Chinese gastric cancer patients. BMC Cancer, 2014, 14: 823.
16.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer(ToGA): a phase3, open-label, randomized controlled trial. Lancet, 2010, 376: 687-697.
17.Gong J, Liu T, Fan Q, et al. Optimal regimen of trastuzumab in combination with oxaliplatin/capecitabine in first-line treatment of HER2-positive advanced gastric cancer(CGOG1001): a multicenter,phaseⅡ trial. BMC Cancer,2016,16(1): 68.
18.Chua C,Tan IB,Yamada Y,et al. PhaseⅡ study of trastuzumab in combination with S-1 and cisplatin in the first-line treatment of human epidermal growth factor receptor HER2-positive advanced gastric cancer. Cancer Chemother Pharmacol, 2015, 76(2): 397-408.
19.HER2阳性晚期胃癌分子靶向治疗的中国专家共识 (2016).临床肿瘤学杂志,2016,21(9):831-839.
20.Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized,phaseⅢ study. J Clin Oncol, 2014, 32(19): 2039-2049.
21.Hecht JR, Bang YJ, Qin SK, et al. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2-Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC-A Randomized Phase Ⅲ Trial. J Clin Oncol,2016, 34(5): 443-451.
22.Kang YK, Shah MA, Ohtsu A, et al. A randomized, open-label, multicenter, adaptive phase 2/3 study of trastuzumab emtansine(T-DM1)versus a taxane(TAX)in patients(pts)with previously treated HER2-positive locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma(LA/MGC/GEJC).J Clin Oncol, 2016, 34(Suppl 4): a5.
23.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma(REGARD): an international, randomized, multicenter, placebo-controlled, phase 3 trial. Lancet, 2014, 383(9911): 31-39.
24.Wilke H,Muro K,Van Cutsem E,et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma(RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol, 2014, 15: 1224-1235.
25.Li J, Qin S, Xu J, et al. Randomized, Double-Blind, Placebo-Controlled Phase Ⅲ Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastro-esophageal Junction. J Clin Oncol, 2016, 34(13): 1448-1454.
26.Ohtsu A, Shah MA,Van Cutsem E,et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase Ⅲ study. J Clin Oncol, 2011, 29(30): 3968-3976.
27.阿帕替尼治疗胃癌的临床应用专家共识.临床肿瘤学杂志,2015,2(9):841-847.
28.Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer(KEYNOTE-012): a multicentre, open-label, phase Ib trial. Lancet Oncol, 2016,17(6):717-726.
29.Le DT, Bendell JC, Calvo E, et al. Safety and activity of nivolumab monotherapy in advanced and metastatic(A/M)gastric or gastroesophageal junction cancer(GC/GEC):results from the CheckMate-032 study. J Clin Oncol, 2016(suppl 4S; abstr 6).
30.Yoon-Koo Kang, Taroh Satoh, Min-Hee Ryu, et al. Nivolumab(ONO-4538/BMS-936558)as salvage treatment after second or later-line chemotherapy for advanced gastric or gastro-esophageal junction cancer(AGC): A double-blinded, randomized, phase Ⅲ trial. J Clin Oncol 35, 2017(suppl 4S;abstract 2).
31.石汉平,李苏宜,王昆华,et al.胃癌患者营养治疗指南.肿瘤代谢与营养电子杂志,2015(2):37-40.
2.2.2 复发或转移性胃癌单一远处转移的综合治疗
1.Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: The new era of cancer therapy. Jpn J Clin Oncol, 2010, 40: 107-111.
2.Milano MT,Katz AW,Zhang H,Okunieff P. Oligometastases treated with stereotactic body radiotherapy: Long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys, 2012, 83: 878-886.
3.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol, 1995, 13: 8-10.
2.2.2.1 术后局部复发或单一远处转移胃癌的治疗
1.Badgwell B, Cormier JN, Xing Y, et al. Attempted salvage resection for recurrent gastric orgastroesophageal cancer. Ann Surg Oncol, 2009, 16(1): 42-50.
2.Xu C, Xie J, Liang N, et al. Concurrent involved-field radiotherapy and XELOX in gastric cancer patients with postoperative oligometastatic recurrence. J Cancer Res Ther, 2014, 10 Suppl: 267-271.
3.Yuan ST, Wang FL, Liu N, et al. Concurrent involved-field radiotherapy and XELOX versus XELOX chemotherapy alone in gastric cancer patients with postoperative locoregional recurrence. Am J Clin Oncol,2015, 38(2): 130-134.
4.Xie J, Liang N, Qiao L, et al. Docetaxel, capecitabine and concurrent radiotherapy for gastric cancer patients with postoperative locoregional recurrence. Tumori, 2015, 101(4): 433-439.
5.Choi SB, Song J, Kang CM, et al. Surgical outcome of metachronous hepatic metastases secondary togastric cancer. Hepatogastroenterology, 2010, 57(97): 29-34.
6.Shinohara T, Maeda Y, Hamada T, Futakawa N. Survival benefit of surgical treatment for liver metastases from gastric cancer. J Gastrointest Surg, 2015, 19(6): 1043-1051.
7.Hwang JE, Kim SH, Jin J, et al. Combination of percutaneous radiofrequency ablation and systemic chemotherapy are effective treatment modalities for metachronous liver metastases from gastric cancer. Clin Exp Metastasis, 2014, 31(1): 25-32.
8.Feng Q, Pei W, Zheng ZX,Bi JJ,Yuan XH. Clinicopathologic characteristics and prognostic factors of 63 gastric cancer patients with metachronous ovarian metastasis. Cancer Biol Med, 2013, 10(2):86-91.
9.Rosa F, Marrelli D, Morgagni P, et al. Krukenberg Tumors of Gastric Origin: The Rationale of Surgical Resection and Perioperative Treatments in a Multicenter Western Experience. World J Surg, 2016, 40(4):921-928.
2.2.2.2 初诊Ⅳ期单一远处转移胃癌的治疗
1.Japanese Gastric CancerAssociation. Japanese classification of gastric carcinoma.3rd. English edition.
2.Kodera Y, Ito S, Mochizuki Y, Ohashi N, et al. Long-term follow up of patients who were positive for peritoneal lavage cytology: final report from the CCOG0301 study. Gastric Cancer, 2012, 15(3): 335-337.
3.Badgwell B, Cormier JN, Krishnan S, et al. Does neoadjuvant treatment for gastric cancer patients with positive peritoneal cytology at staging laparoscopy improve survival?Ann Surg Oncol, 2008, 15(10):2684-2691.
4.Okabe H, Ueda S, Obama K, et al. Induction chemotherapy with S-1 plus cisplatin followed by surgery for treatment of gastric cancer with peritoneal dissemination. Ann Surg Oncol, 2009, 16 (12):3227-3236.
5.Yamamoto M,Kawano H,Yamaguchi S,et al. Comparison of Neoadjuvant Chemo-therapy to Surgery Followed by Adjuvant Chemotherapy in Japanese Patients with Peritoneal Lavage Cytology Positive for Gastric Carcinoma. Anticancer Res, 2015 Sep, 35(9): 4859-4863.
6.Masuda T, Kuramoto M, Shimada S, et al. The effect of extensive intraoperative peritoneal lavage therapy(EIPL)on stageⅢ B+C and cytology-positive gastric cancer patients. Int J Clin Oncol, 2016, 21(2):289-294.
7.Satoh S, Okabe H, Teramukai S, et al. PhaseⅡtrial of combined treatment consisting of preoperative S-1 plus cisplatin followed by gastrectomy and postoperative S-1 for stage Ⅳ gastric cancer. Gastric Cancer,2012, 15(1): 61-69.
8.Tsuburaya A, Mizusawa J, Tanaka Y, et al. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg, 2014, 101(6): 653-660.
9.Wang Y,Yu YY,Li W,et al. A phaseⅡ trial of Xeloda and oxaliplatin(XELOX)neo-adjuvant chemotherapy followed bysurgery for advanced gastric cancer patients with para-aortic lymph node metastasis. Cancer Chemother Pharmacol, 2014, 73(6): 1155-1161.
10.Fujitani K, Yang HK, Mizusawa J, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor(REGATTA): phase 3, randomised controlled trial. Lancet Oncol, 2016, 17(3): 309-318.
11.Markar SR, Mackenzie H, Mikhail S, et al. Surgical resection of hepatic metastases from gastric cancer:outcomes from national series in England. Gastric Cancer, 2016, 20(2): 379-386.
12.Kinoshita T, Kinoshita T, Saiura A, et al. Multicentre analysis of long-term outcome after surgical resection forgastric cancer liver metastases. Br J Surg, 2015, 102(1): 102-107.
13.Shinohara T, Maeda Y, Hamada T, et al. Survival benefit of surgical treatment for liver metastases from gastric cancer. J Gastrointest Surg, 2015, 9(6): 1043-1051.
14.Petrelli F, Coinu A, Cabiddu M, et al. Hepatic resection for gastric cancer liver metastases: A systematic review and meta-analysis. J Surg Oncol, 2015, 111(8): 1021-1027.
15.Grimes N, Devlin J, Dunne DF, et al. The role of hepatectomy in the management of metastatic gastric adenocarcinoma: a systematic review. Surg Oncol, 2014, 23(4): 177-185.
16.Martella L, Bertozzi S, Londero AP, et al. Surgery for Liver Metastases From Gastric Cancer: A Meta-Analysis of Observational Studies. Medicine(Baltimore),2015,94(31): e1113.
17.Tiberio GA, Baiocchi GL, Morgagni P, et al. Gastric cancer and synchronous hepatic metastases: is it possible to recognize candidates to R0 resection?Ann Surg Oncol, 2015, 22(2): 589-596.
18.Tiberio GA,Ministrini S, Gardini A,et al. Factors influencing survival after hepatectomy for metastases fromgastric cancer. Eur J Surg Oncol,2016, 42(8):1229-1235.
19.Jiang H,Li Q,Yu S, et al. Impact of HER2 expression on outcome in gastric cancer patients withliver metastasis. Clin Transl Oncol, 2017 , 19(2): 197-203.
20.Kim HR,Cheon SH, Lee KH, et al. Efficacy and feasibility of radiofrequency ablation for liver metastases from gastric adenocarcinoma. Int J Hyperthermia, 2010, 26(4): 305-315.
21.Chen J, Tang Z, Dong X, et al. Radiofrequency ablation for liver metastasis from gastric cancer. Eur J Surg Oncol, 2013, 39(7): 701-706.
22.Peng W, Hua RX, Jiang R, et al. Surgical treatment for patients with Krukenberg tumor of stomach origin:clinical outcome and prognostic factors analysis. PLoS One, 2013, 8(7): e68227.
23.Brieau B, Auzolle C, Pozet A, et al. Efficacy of modern chemotherapy and prognostic factors in patients with ovarian metastases from gastric cancer: A retrospective AGEO multicentrestudy. Dig Liver Dis,2016, 48(4): 441-445.
24.Cho JH, Lim JY, Choi AR, et al. Comparison of surgery plus chemotherapy and palliative chemotherapy alone for advanced gastric cancer with Krukenberg tumor. Cancer Res Treat, 2015, 47(4):697-705.
25.Lu LC, Shao YY, Hsu CH, et al. Metastasectomy of Krukenberg tumors may be associated with survival benefits in patients with metastatic gastric cancer. Anticancer Res, 2012, 32(8): 3397-3401.
3 随访
1.Smyth EC, Verheij M, Allum W, et al. Gastric cancer:ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. ANN ONCOL, 2016, 27(suppl 5): v38-49.
2.Ajani JA, D’ Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw, 2016, 14(10): 1286-1312.
3.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014(ver.4).GASTRIC CANCER, 2017, 20(1): 1-19.
4.3 胃癌cT分期征象及报告参考
1.Seevaratnam R, Cardoso R, Mcgregor C, et al.How useful is preoperative imaging for tumor, node,metastasis(TNM)staging of gastric cancer?A meta-analysis.Gastric Cancer, 2012, 15 Suppl 1(1):S3-18.
2.Kumano S, Murakami T, Kim T, et al.T staging of gastric cancer: role of multi-detector row CT.Radiology, 2005, 237(3): 961-966.
3.Kim JW,Shin SS,Heo SH,et al.Diagnostic performance of 64-section CT using CT gastrography in preoperative T staging of gastric cancer according to 7th edition of AJCC cancer staging manual.Eur Radiol, 2012, 22(3): 654-662.
4.Hasegawa S, Yoshikawa T, Shirai J, et al.A prospective validation study to diagnose serosalinvasionand nodal metastases of gastric cancer by multidetector-row CT.Ann SurgOncol, 2013 , 20(6):2016-2022.
5.Habermann CR, Weiss F, Riecken R, et al.Preoperative stagingof gastric adeno-carcinoma: comparison of helical CT and endoscopicUS.Radiology, 2004, 230(2):465-471.
6.Kim TU, Kim S, Lee JW,et al.MDCT features in the differentiation of T4a gastric cancerfrom less-advanced gastric cancer: significance of the hyper-attenuating serosa sign.Br J Radiol, 2013 , 86(1029):20130290.
7.Lee SL, Ku YM, Jeon HM, et al.Impact of the cross-sectional location of multidetector computed tomography scans on prediction of serosalexposurein patients with advanced gastric cancer.Ann Surg Oncol,2017 , 24(4): 1003-1009.
8.Amin MB, Edge SB, Greene FL, et al, eds.AJCC Cancer Staging Manual.8th ed.New York:Springer,2017.
9.Fukuya T, Honda H, Hayashi T, et al.Lymphnode metastases: efficacy for detection with helical CT in patients with gastric cancer.Radiology, 1995 , 197(3): 705-711.
10.Robert MK, Thomas CK.Imaging in assessing lymph node status in gastric cancer.Gastric Cancer,2009,12:6-22.
4.4 胃癌超声内镜(EUS)分期征象
1.Barbour AP, Rizk NP,Gerdes H,et al.Endoscopic ultrasound predicts outcomes for patients with adenocarcinoma of the gastroesophageal junction.Journal of the American College of Surgeons,2007,205(4):593-601.
2.Blackshaw G, Lewis WG, Hopper AN, et al.Prospective comparison of endosonography, computed tomography, and histopathological stage of junctional oesphagogastric cancer.Clin Radiol, 2008 , 63(10):1092-1098.
3.Murata Y,Napoleon B,Odegaard S.High-frequency endoscopic ultrasonography in evaluation of superficial esophageal cancer.Endoscopy, 2003, 35(5): 429-435, discussion 436.
4.Mocellin S,Pasquali S.Diagnostic accuracy of endoscopic ultrasonography(EUS)for the preoperative locoregional staging of primary gastric cancer.Cochrane Database Syst Rev, 2015, 2: CD009944.
5.Catalano MF, Sivak MV, Jr., Rice T, Gragg LA, Van Dam J.Endosonographic features predictive of lymph node metastasis.Gastrointestinal endoscopy, 1994, 40(4): 442-446.
6.Puli SR, Reddy JB, Bechtold ML, et al.Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systemic review.World journal of gastroenterology: WJG, 2008, 14(10):1479-1490.
7.tenBerge J, Hoffman BJ, Hawes RH, et al.EUS-guided fine needle aspiration of liver: indications,yield, and safety based on an international survey of 167 cases.Gastrointestinal endoscopy,2002 , 55(7):859-862.
8.Lee YT, Ng EK, Hung LC, et al.Accuracy of endoscopic ultrasonography in diagnosing ascites and predicting peritoneal metastasis in gastric cancer patients.Gut, 2005, 54(11): 1541-1545.
9.Sultan J, Robinson S, Hayes N, et al.Endoscopic ultrasonography-detected low-volume ascites as a predictor of inoperability for osephagogastric cancer.The British journal of surgery, 2008, 95(9):1127-1130.