 去看看
去看看


据统计中国每年超过30万人死于肝细胞癌(以下简称肝癌),占全球肝癌死亡人数的一半左右。而肝移植是被全世界认可的治疗终末期肝病的有效手段之一。我国自20世纪90年代掀起第2次肝移植热潮以来,肝移植事业发展迅猛,呈专业化和规模化发展态势,在移植数量和质量方面已接近或达到西方发达国家水平。截至2014年4月,中国肝移植注册网站登记肝移植26751例。目前,肝移植在全国范围内已得到广泛开展,亟待相关临床实践指南来指导全国肝移植工作更规范、安全、有效地开展。中华医学会器官移植学分会、中华医学会外科学分会移植学组及中国医师协会器官移植医师分会组织专家制订了《中国肝癌肝移植临床实践指南(2014版)》(以下简称“指南”),重点阐述肝移植受者选择标准、术前降期治疗、受者抗病毒治疗、受者免疫抑制剂应用、术后肿瘤复发的防治5部分内容。
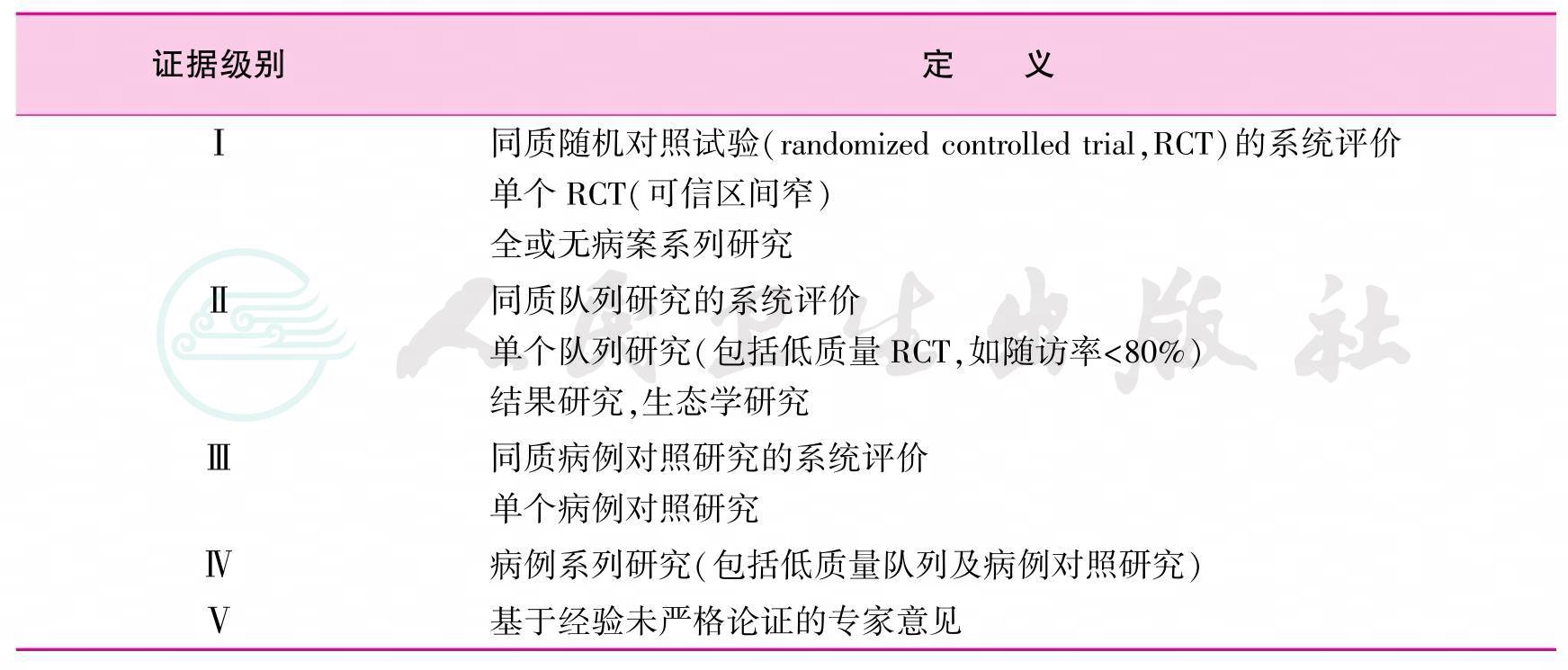
1 循证医学证据
本指南采用的循证医学证据分级主要参考2001年牛津大学循证医学中心证据分级标准(表1),推荐意见强度主要参考GRADE系统推荐分级等[1-2]。
表1 循证医学证据分级标准
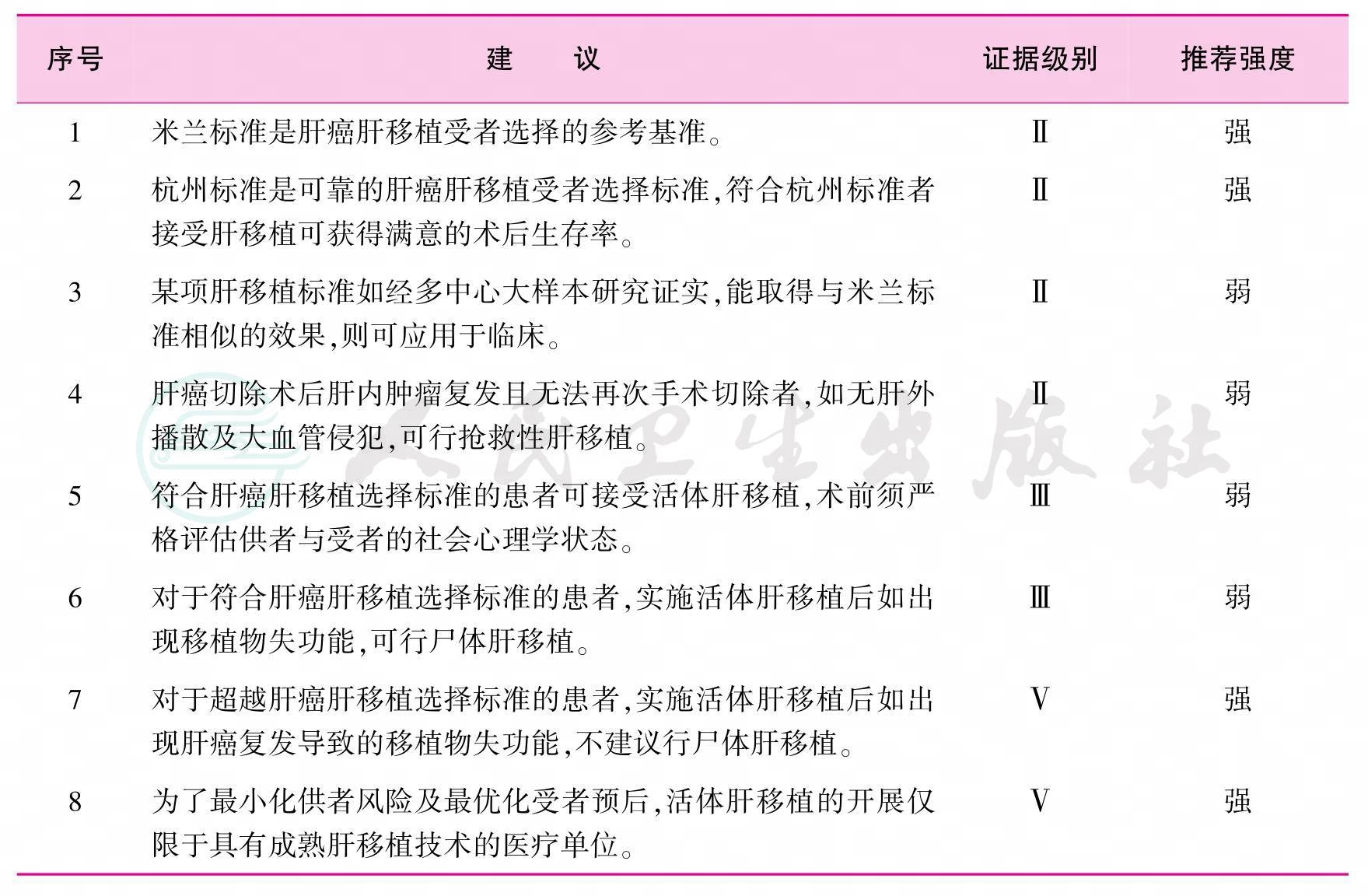
2 肝癌肝移植受者选择标准(表2)
供肝短缺是世界性难题,故应将宝贵的供肝资源优先分配给肝移植的最大获益者。心脏死亡器官捐献是中国目前拓展供肝来源的主要方向,而活体肝移植在有丰富移植经验的医疗单位已成为一项成熟技术[3]。1996年,Mazzaferro等提出米兰标准后,符合米兰标准的肝癌肝移植受者获得了长期生存[4-7]。但米兰标准对肝癌大小和数目的限制过于严格,更重要的是忽略了肿瘤的生物学特性。如果根据米兰标准,中国大多数肝癌患者将失去肝移植机会。近年来国际上涌现出一些新的肝癌肝移植受者选择标准,如加州大学旧金山分校(UCSF)标准、Up-to-Seven标准等,这些新标准提出的共同目的是扩大受者人群并取得与米兰标准相似的移植生存率[8-9]。2008年,中国提出的杭州标准是国际上率先引入肿瘤生物学特性和病理学特征的肝移植标准,这是对以往局限于肿瘤形态学标准的巨大突破。研究结果证实:无论是尸体肝移植还是活体肝移植,符合杭州标准的肝移植受者均获得满意的术后生存率[10-15]。近年来,对于肝癌切除术后复发者,如符合肝移植准入标准,多数专家主张行抢救性肝移植;对于肝癌肝移植术后移植物失功能者,再次肝移植应审慎考虑[16-17]。
表2 肝癌肝移植受者选择标准
3 肝癌肝移植术前降期治疗(表3)
肝癌肝移植术前肿瘤降期治疗是通过一系列治疗手段,减轻肿瘤负荷,降低分期,使不满足肝癌肝移植受者选择标准的患者能够被纳入移植标准,获得肝移植机会。降期治疗主要适用于不符合现有肝癌肝移植标准,且无门静脉主干或下腔静脉等大血管侵犯、无远处转移的肝癌患者[18-21]。降期治疗的方法主要有局部消融治疗和TACE等[18-19,22]。局部消融治疗包括RFA、微波消融、冷冻消融和经皮无水乙醇注射等方法。降期治疗的疗效采用增强CT和MRI检查结合AFP进行评估,评价指标包括肿瘤大小、数目和AFP水平等[22-28]。目前有研究结果显示:多种治疗方法的联合应用可达到更好的降期疗效[29]。
表3 肝癌肝移植术前降期治疗

4 肝癌肝移植受者抗病毒治疗(表4)
中国肝癌肝移植受者90%以上与HBV感染相关。肝移植前HBV载量高以及肝移植后乙型病毒性肝炎(以下简称乙肝)复发的受者,肝癌复发的风险增加,因此,对乙肝肝移植受者尽早行抗病毒治疗,尽快降低HBV水平,有助于降低肝移植术后乙型肝炎复发率,提高受者长期生存率[30-32]。HBV载量高的等待肝移植患者应采用恩替卡韦等强效、高耐药屏障核苷类似物(nucleostide analogues,NAs)。肝移植术中无肝期应给予乙肝免疫球蛋白(hepatitis B immunoglobulin,HBIG)。肝移植术后的主要抗病毒治疗方案为NAs联合低剂量HBIG,其中恩替卡韦或替诺福韦的联合方案能更好地预防移植术后乙肝复发[33-38]。应用无激素免疫抑制方案可降低移植术后乙肝复发率[39]。此外也有肝移植患者术后接种乙肝疫苗预防乙肝复发的报道,其临床应用尚有争议[40-42]。中国HCV感染患者呈增多趋势,HCV RNA阳性患者如肝功能Child-Pugh评分≤7分,术前宜进行抗病毒治疗,移植术后须经病理检查确认丙型病毒性肝炎复发后方可给予抗HCV治疗[43]。
表4 肝癌肝移植受者抗病毒治疗

注:NAs:高耐药屏障核苷类似物;HBIG:乙肝免疫球蛋白
5 肝癌肝移植受者免疫抑制剂应用(表5)
钙调磷酸酶抑制剂(calcineurin inhibitor,CNI)的应用是肝移植后肝癌复发的独立危险因素[44]。对于肝癌肝移植受者,肿瘤的复发风险与其侵袭性及机体的免疫功能有关,受者处于强免疫抑制状态时其免疫监视系统受到破坏,促进肿瘤复发、转移,而免疫抑制剂量不足则容易诱发排斥反应。如何维持这一平衡,目前尚无定论[45-47]。肝癌肝移植受者目前尚不建议将免疫抑制剂全线撤除,但主张个体化的低剂量免疫抑制方案[45]。近年来临床上有糖皮质激素早期撤除、无糖皮质激素及使用具有肿瘤抑制作用的mTOR抑制剂(西罗莫司为代表)的成功应用方案[44,48-50]。目前临床上主要的免疫抑制方案为:(1)他克莫司或环孢素+吗替麦考酚酯+糖皮质激素;(2)IL-2受体阻滞剂+西罗莫司+吗替麦考酚酯+糖皮质激素;(3)IL-2受体阻滞剂+吗替麦考酚酯+他克莫司/西罗莫司[51-54]。
表5 肝癌肝移植受者免疫抑制剂应用

注:CNI:钙调磷酸酶抑制剂
6 肝癌肝移植术后肿瘤复发的防治(表6)
肝癌肝移植术后5年肝癌复发率可达20.0%~57.8%,故复发、转移的防治十分重要[9,55]。肝癌的形态学特征(大小、数目等)、分期、组织学分级以及生物学特性等应作为术后用药的重要参考,制订个体化治疗方案。
表6 肝癌肝移植术后复发的防治

肝癌肝移植术后可能存在针对肿瘤的免疫逃逸,故应给予受者一定疗程的术后治疗,以期尽可能地减少微小转移灶,降低术后复发率。选用碘131美妥昔单抗放射免疫治疗、索拉非尼治疗以及系统性化疗(如奥沙利铂或阿霉素分别与氟尿嘧啶联合使用),均可为部分受者提供一定的生存获益[56-59]。
对于肝移植术后肝癌复发转移者,应用索拉非尼治疗,可延长受者生存时间[18,60-62]。肺转移灶如可切除,首选手术切除[63]。移植肝内复发病灶的局部治疗包括手术切除、TACE、局部消融等[64-66]。有专家提出放疗、再次肝移植等可作为治疗的选择。对于晚期患者,可考虑减少或停止免疫抑制剂的使用。
[1]Oxford Centre for Evidence-based Medicine—Levels of Evidence(March 2009)[DB/OL].Oxford Centre for Evidence-based Medicine,2009[2014-04-15].http://www.cebm.net/oxford-centre-evidence-based-medicinelevels-evidence-march-2009/.
[2]Schünemann HJ,Oxman AD,Brozek J,et al.Grading quality of evidence and strength of recommendations for diagnostic tests and strategies[J].BMJ,2008,336(7653):1106-1110.
[3]Liang WH,Wu LW,Ling XT,et al.Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma:a meta-analysis[J].Liver Transpl,2012,18(10):1226-1236.
[4]Mazzaferro V,Regalia E,Doci R,et al.Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis[J].N Engl J Med,1996,334(11):693-699.
[5]European Liver Transplant Registry Results[DB/OL].European Liver Transplant Registry,2011[2014-04-15].http://www.eltr.org.
[6]OPTN/SRTR Annual Report[DB/OL].Organ Procurement and Transplantation Network,2011[2014-04-15]. http://www.ustransplant.org.
[7]Mazzaferro V,Bhoori S,Sposito C,et al.Milan criteria in liver transplantation for hepatocellular carcinoma:an evidence-based analysis of 15 years of experience[J].Liver Transpl,2011,17(Suppl 2):S44-57.
[8]Yao FY,Ferrell L,Bass NM,et al.Liver transplantation for hepatocellular carcinoma:expansion of the tumor size limits does not adversely impact survival[J].Hepatology,2001,33(6):1394-1403.
[9]Mazzaferro V,Llovet JM,Miceli R,et al.Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria:a retrospective,exploratory analysis[J].Lancet Oncol,2009,10(1):35-43.
[10]Zheng SS,Xu X,Wu J,et al.Liver transplantation for hepatocellular carcinoma:Hangzhou experiences[J]. Transplantation,2008,85(12):1726-1732.
[11]Lei JY,Wang WT,Yan LN.Hangzhou criteria for liver transplantation in hepatocellular carcinoma:a singlecenter experience[J].Eur J Gastroenterol Hepatol,2014,26(2):200-204.
[12]Audet M,Panaro F,Piardi T,et al.Are the Hangzhou criteria adaptable to hepatocellular carcinoma patients for liver transplantation in Western countries?[J].Liver Transpl,2009,15(7):822-826.
[13]Chen J,Xu X,Wu J,et al.The stratifying value of hangzhou criteria in liver transplantation for hepatocellular carcinoma[J].PLoS One,2014,9(3):e93128.
[14]徐骁,杨家印,钟林,等.肝癌肝移植“杭州标准”多中心应用研究——1163例报道[J].中华器官移植杂志,2013,34(9):524-527.
[15]郑树森,汪恺,徐骁,等.肝移植治疗肝癌的受者选择杭州标准在亲属活体供肝移植中的应用价值[J].中华器官移植杂志,2011,32(6):330-333.
[16]Hu ZH,Wang W,Li ZW,et al.Recipient outcomes of salvage liver transplantation versus primary liver transplantation:a systematic review and meta-analysis[J].Liver Transpl,2012,18(11):1316-1323.
[17]Olthoff KM,Merion RM,Ghobrial RM,et al.Outcomes of 385 adult-to-adult living donor liver transplant recipients:a report from the A2ALL Consortium[J].Ann Surg,2005,242(3):314-325.
[18]Clavien PA,Lesurtel M,Bossuyt PM,et al.Recommendations for liver transplantation for hepatocellular carcinoma:an international consensus conference report[J].Lancet Oncol,2012,13(1):e11-22.
[19]Galuppo R,McCall A,Gedaly R.The role of bridging therapy in hepatoeellular carcinoma[J].Int J Hepatol,2013:419302.
[20]European Association for the Study of the Liver.EASL-EORTC clinical practice guidelines:management of hepatocellular carcinoma[J].J Hepatol,2012,56(4):908-943.
[21]Washburn K,Edwards E,Harper A,et al.Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system[J].Am J Transplant,2010,10(7):1643-1648.
[22]Lewandowski RJ,Kulik LM,Riaz A,et al.A comparative analysis of transarterial down staging for hepatocellular carcinoma:chemoembolization versus radioembolization[J].Am J Transplant,2009,9(8):1920-1928.[23]Merani S,Majno P,Kneteman NM,et al.The impact of waiting list alpha-fetoprotein changes on the outcome of liver transplant for hepatocellular carcinoma[J].J Hepatol,2011,55(4):814-819.
[24]Mailey B,Artinyan A,Khalili J,et al.Evaluation of absolute serum α-fetoprotein levels in liver transplant for hepatocellular cancer[J].Arch Surg,2011,146(1):26-33.
[25]Ravaioli M,Grazi GL,Piscaglia F,et al.Liver transplantation for hepatocellular carcinoma:results of downstaging in patients initially outside the Milan selection criteria[J].Am J Transplant,2008,8(12):2547-2557.
[26]Yao FY,Kerlan RK Jr,Hirose R,et al.Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation:an intention-to-treat analysis[J].Hepatology,2008,48(3):819-827.
[27]Duvoux C,Roudot-Thoraval F,Decaens T,et al.Liver transplantation for hepatocellular carcinoma:a model including α-fetoprotein improves the performance of Milan criteria[J].Gastroenterology,2012,143(4):986-994.
[28]Lai Q,Avolio AW,Graziadei I,et al.Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation[J].Liver Transpl,2013,19(10):1108-1118.
[29]Ashoori N,Bamberg F,Paprottka P,et al.Multimodality treatment for early-stage hepatocellular carcinoma:a bridging therapy for liver transplantation[J].Digestion,2012,86(4):338-348.
[30]Koda M,Nagahara T,Matono T,et al.Nucleotide analogs for patients with HBV-related hepatocellular carcinoma increase the survival rate through improved liver function[J].Inter Med,2009,48(1):11-17.
[31]Wong JS1,Wong GL,Tsoi KK,et al.Meta-analysis:the efficacy of anti-viral therapy in prevention of recurrence after curative treatment of chronic hepatitis B-related hepatocellular carcinoma[J].Aliment Pharmacol Ther,2011,33(10):1104-1112.
[32]Wu JC,Huang YH,Chau GY,et al.Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma[J].J Hepatol,2009,51(5):890-897.
[33]European Association for the Study of the Liver.EASL clinical practice guidelines:Management of chronic hepatitis B virus infection[J].J Hepatol,2012,57(1):167-185.
[34]Chen CJ,Yang HI,Iloeje UH,et al.Hepatitis B virus DNA levels and outcomes in chronic hepatitis B[J]. Hepatology,2009,49(Suppl 5):S72-84.
[35]Lai CL,Yuen MF.Prevention of hepatitis B virus-related hepatocellular carcinoma with antiviral therapy[J]. Hepatology,2013,57(1):399-408.
[36]Yin JH,Li N,Han YF,et al.Effect of antiviral treatment with nucleotide/nucleoside analogs on postoperative prognosis of hepatitis B virus-related hepatocellular carcinoma:a two-stage longitudinal clinical study[J].J Clin Oncol,2013,31(29):3647-3655.
[37]Huang G,Lai EC,Lau WY,et al.Posthepatectomy HBV reactivation in hepatitis B-related hepatocellular carcinoma influences postoperative survival in patients with preoperative low HBV-DNA levels[J].Ann Surg,2013,257(3):490-505.
[38]Hu TH,Chen CL,Lin CC,et al.Section 14.Combination of entecavir plus low-dose on-demand hepatitis B immunoglobulin is effective with very low hepatitis B recurrence after liver transplantation[J].Transplantation,2014,97(Suppl 8):S53-59.
[39]Kim JM,Joh JW,Kim SJ,et al.Steroid withdrawal in adult liver transplantation:occurrence at a single center [J].Transplant Proc,2010,42(10):4132-4136.
[40]Sánchez-Fueyo A,Rimola A,Grande L,et al.Hepatitis B immunoglobulin discontinuation followed by hepatitis B virus vaccination:A new strategy in the prophylaxis of hepatitis B virus recurrence after liver transplantation[J].Hepatology,2000,31(2):496-501.
[41]Lo CM,Liu CL,Chan SC,et al.Failure of hepatitis B vaccination in patients receiving lamivudine prophylaxis after liver transplantation for chronic hepatitis B[J].J Hepatol,2005,43(2):283-287.
[42]Lo CM,Lau GK,Chan SC,et al.Efficacy of a pre-S containing vaccine in patients receiving lamivudine prophylaxis after liver transplantation for chronic hepatitis B[J].Am J Transplant,2007,7(2):434-439.
[43]Watt K,Veldt B,Charlton M.A practical guide to the management of HCV infection following liver transplantation[J].Am J Transplant,2009,9(8):1707-1713.
[44]Vivarelli M,Cucchetti A,La Barba G,et al.Liver transplantation for hepatocellular carcinoma under calcineurin inhibitors:reassessment of risk factors for tumor recurrence[J].Ann Surg,2008,248(5):857-862.
[45]Chen K,Man K,Metselaar HJ,et al.Rationale of personalized immunosuppressive medication for hepatocellular carcinoma patients after liver transplantation[J].Liver Transpl,2014,20(3):261-269.
[46]Miyagi S,Kawagishi N,Sekiguchi S,et al.The relationship between recurrences and immunosuppression on living donor liver transplantation for hepatocellular carcinoma[J].Transplant Proc,2012,44(3):797-801.
[47]Saigal S,Shah S.Liver transplantation-economics in the less developed world[J].Indian J Gastroenterol,2012,31(1):13-14.
[48]Foroncewicz B,Mucha K,Ryszkowska E,et al.Safety and efficacy of steroid-free immunosuppression with tacrolimus and daclizumab in liver transplant recipients:6-year follow-up in a single center[J].Transplant Proc,2009,41(8):3103-3106.
[49]Nair S,Eason J,Loss G.Sirolimus monotherapy in nephrotoxicity due to calcineurin inhibitors in liver transplant recipients[J].Liver Transpl,2003,9(2):126-129.
[50]Menon KV,Hakeem AR,Heaton ND.Meta-analysis:recurrence and survival following the use of sirolimus in liver transplantation for hepatocellular carcinoma[J].Aliment Pharmacol Ther,2013,37(4):411-419.
[51]Liang W,Wang D,Ling X,et al.Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma:a meta-analysis[J].Liver Transpl,2012,18(1):62-69.
[52]Soliman T,Hetz H,Burghuber C,et al.Short-term induction therapy with anti-thymocyte globulin and delayed use of calcineurin inhibitors in orthotopic liver transplantation[J].Liver Transplant,2007,13(7):1039-1044.
[53]Martin-Mateos RM,Graus J,Albillos A,et al.Initial immunosuppression with or without basiliximab:a comparative study[J].Transplant Proc,2012,44(9):2570-2572.
[54]Pillai AA,Levitsky J.Overview of immunosuppression in liver transplantation[J].World J Gastroenterol,2009,15(34):4225-4233.
[55]Zimmerman MA,Ghobrial RM,Tong MJ,et al.Recurrence of hepatocellular carcinoma following liver transplantation:a review of preoperative and postoperative prognostic indicators[J].Arch Surg,2008,143(2):182-188.
[56]Xu J,Shen ZY,Chen XG,et al.A randomized controlled trial of Licartin for preventing hepatoma recurrence after liver transplantation[J].Hematology,2007,5(2):269-276.
[57]Huang L,Li GM,Zhu JY,et al.Efficacy of sorafenib after liver transplantation in patients with primary hepatic carcinoma exceeding the Milan criteria:a preliminary study[J].Oncol Targets Ther,2012,5:457-462.
[58]Zhang Q,Chen H,Li Q,et al.Combination adjuvant chemotherapy with oxaliplatin,5-fluorouracil and leucovorin after liver transplantation for hepatocellular carcinoma:a preliminary open-label study[J].Invest New Drugs,2011,29(6):1360-1369.
[59]张照辉,马力文,宋世兵,等.肝癌肝移植术后辅助化疗的临床分析[J].中华肿瘤杂志,2005,27(1):45-47.
[60]Waghray A,Balci B,El-Gazzaz G,et al.Safety and efficacy of sorafenib for the treatment of recurrent hepatocellular carcinoma after liver transplantation[J].Clin transplant,2013,27(4):555-561.
[61]Pfeiffenberger J,Koschny R,Hoffmann K,et al.Sorafenib treatment is save and may affect survival of recurrent hepatocellular carcinoma after liver transplantation[J].Langenbceks Arch Surg,2013,398(8):1123-1128.
[62]Sposito C,Mariani L,Germini A,et al.Comparative efficacy of sorafenib versus best supportive care in recurrent hepatocellular carcinoma after liver transplantation:a case-control study[J].J Hepatol,2013,59(1):59-66.
[63]Hwang S,Kim YH,Kim DK,et al.Resection of pulmonary metastases from hepatocellular carcinoma following liver transplantation[J].World J Surg,2012,36(7):1592-1602.
[64]Taketomi A,Fukuhara T,Morita K,et al.Improved results of a surgical resection for the recurrence of hepatocellular carcinoma after living donor liver transplantation[J].Ann Surg Oncol,2010,17(9):2283-2289.
[65]Ko HK,Ko GY,Yoon HK,et al.Tumor response to transcatheter arterial chemoembolization in recurrent hepatocellular carcinoma after living donor liver transplantation[J].Korean J Radiol,2007,8(4):320-327.
[66]Ho CK,Chapman WC,Brown DB.Radiofrequency ablation of recurrent hepatocellular carcinoma in a patient after liver transplantation:two-year follow-up[J].J Vasc Interv Radiol,2007,18(11):1451-1453.